What is the Ozone Layer?
The ozone layer is a crucial component of Earth's stratosphere, located between 19 and 30 km above the surface. It plays a vital role in filtering harmful ultraviolet (UV) radiation from the sun, thus safeguarding life on Earth.
.jpg)
Source:ourworldindata/Hannah Ritchie
Functions of the Ozone Layer
- Absorbs UV radiation: Prevents excessive UV-B and UV-C rays from reaching Earth.
- Protects living organisms: Reduces risks such as skin cancer, cataracts, and weakened immune systems.
- Preserves ecosystems: Shields marine life, especially plankton, which is fundamental to the oceanic food chain.
- Prevents material degradation: Protects paints, fabrics, and other materials from UV damage.
Why is the ozone layer important?
Protective Shield Against UV Radiation
The ozone layer acts as Earth’s sunscreen, absorbing 97-99% of the sun’s harmful ultraviolet (UV-B) radiation. Without it, all living beings would be exposed to excessive UV rays, leading to DNA damage, skin cancer, environmental disruption, and affecting marine ecosystems & plant life.
Why is the Southern Hemisphere More Affected by Ozone Depletion?
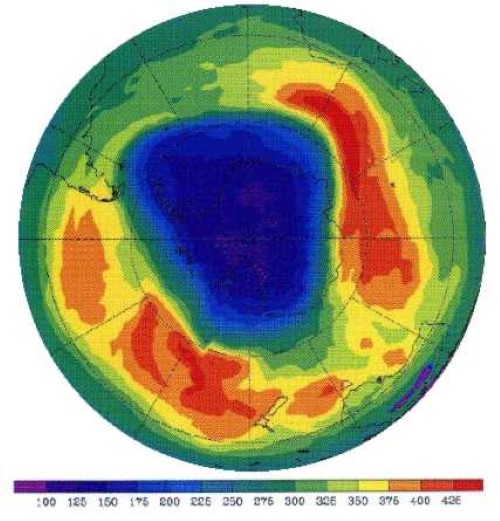
Source:gml.noaa
Studies show that ozone depletion has been more pronounced in the Southern Hemisphere, especially in high-latitude areas. This is due to the presence of colder temperatures, which facilitate the formation of polar stratospheric clouds. These clouds accelerate chemical reactions that break down ozone molecules. In contrast, ozone depletion is minimal near the equator and worsens toward the poles, highlighting the role of temperature and sunlight in this phenomenon.
Why Does the Ozone Hole Form?
- Polar Vortex: A wind pattern traps cold air over Antarctica, preventing ozone-rich air from mixing.
- Stratospheric Clouds: Extreme cold leads to cloud formation, enhancing ozone depletion reactions.
- Springtime Activation: When sunlight returns in September-October, chemical reactions accelerate, causing a dramatic drop in ozone levels.
- Self-repair Mechanism: In summer, new ozone flows in from lower latitudes, temporarily closing the hole.
Effects of Ozone Depletion on UV Radiation
UV radiation is categorised into three types:
| UV Type | Wavelength (nm) | Effects |
| UV-A | 315-400 nm | Least harmful, causes ageing and wrinkles. |
| UV-B | 280-315 nm | Causes skin cancer, cataracts, and immune suppression. |
| UV-C | <280 nm | Highly dangerous but mostly absorbed by the ozone layer. |
Even a minor ozone reduction significantly increases UV-B radiation, heightening the risks to human health and ecosystems.
Ozone Layer Depletion and Its Link to Climate Change
Although ozone depletion and climate change are separate phenomena, they share some interconnected aspects.
| Ozone Depletion | Climate Change |
| Caused by chlorofluorocarbons (CFCs) and other ozone-depleting substances (ODS). | Primarily driven by greenhouse gases (GHGs) like CO2 and methane. |
| Leads to increased UV radiation on Earth’s surface. | Traps heat, causing global warming. |
| Results in health issues, crop damage, and material degradation. | Alter weather patterns melt ice caps and raise sea levels. |
| CFCs, while banned, persist in the atmosphere. | CO2 emissions continue to increase. |
Causes of Ozone Depletion
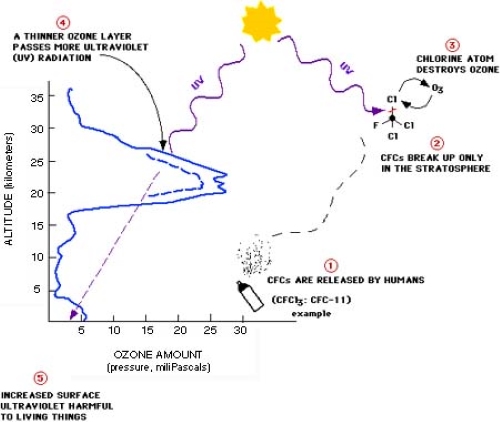
Source:gml.noaa
- Chlorofluorocarbons (CFCs): Found in refrigerants, aerosols, and industrial solvents. They release chlorine atoms that destroy ozone molecules.
- Halons and Bromine Compounds: Used in fire extinguishers and pesticides.
- Nitrous Oxide (N2O): Emitted from fertilisers and fossil fuel combustion, contributing to ozone degradation.
Mechanism of Ozone Destruction
- CFCs break down in the stratosphere due to UV radiation.
- Chlorine atoms are released, which then react with ozone (O3) molecules.
- One chlorine atom can destroy up to 100,000 ozone molecules.
- This process depletes the ozone concentration, allowing more UV radiation to penetrate Earth’s surface.
What Led to Ozone Depletion?
The 1970s-80s Crisis
Human activities release large amounts of ozone-depleting substances (ODS), like CFCs. Ozone levels in the stratosphere dropped significantly. The ozone hole emerged, allowing more UV-B radiation to reach Earth’s surface.
A Global Success Story: Ozone Recovery
The Montreal Protocol (1987) & Global Action Results:
- Phased out ozone-depleting substances.
- The ozone layer is gradually recovering.
- Millions of skin cancer cases were prevented.
One of the biggest international environmental success stories!
Milestones in Ozone Protection
- 1977: GML began monitoring CFCs and ozone-depleting substances.
- 1987: The Montreal Protocol was adopted, phasing out CFC production.
- 1996: Industrialised nations banned CFCs.
- 2020s: Ozone hole shows signs of recovery, but monitoring continues.
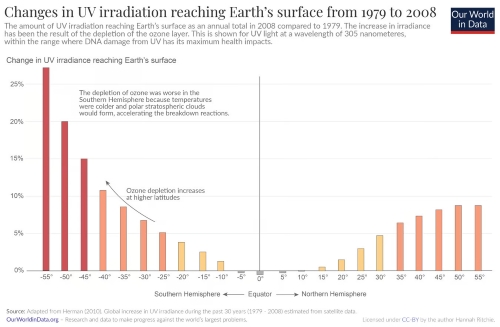
Source:ourworldindata
Ozone Monitoring and Global Efforts
The Global Monitoring Laboratory (GML), part of NOAA, plays a key role in ozone observation.
Ozone Monitoring Methods
- Dobson Spectrophotometers: Measure total ozone levels at 16 global stations.
- Ozonesondes: Weather balloons equipped with sensors track ozone vertical profiles.
- Satellite Observations: Validate ground measurements and track global ozone trends.
- Halocarbon Analysis: Monitors chlorine and bromine compounds that contribute to ozone loss.
Conclusion
The ozone layer remains a critical shield protecting life on Earth. While international efforts like the Montreal Protocol have curbed ozone depletion, the persistence of CFCs means recovery is slow but steady. Ongoing monitoring and adherence to global policies are crucial to restoring and safeguarding the ozone layer for future generations.
Comments
All Comments (0)
Join the conversation