Boyle's Law (Pressure-Volume Relationship): Boyle’s law was formulated by an Irish scientist Robert Boyle in the 17th century to describe the relationship between the pressure and volume of a gas. This law forms the fundamental concept in understanding the behaviour of gases and has numerous applications in various scientific and industrial fields.
Boyle’s Law: Definition
Boyle’s law states that at a constant temperature, the pressure exerted by a gas is inversely proportional to the volume occupied by it. In other words, we can say that volume and pressure are inversely proportional to each other as long as the temperature and amount of gas are kept constant. This means that if you increase the pressure on a gas, its volume will decrease, and vice versa.
Boyle’s Law: Explanation
Boyle's law can be explained using the kinetic theory of gases. According to the kinetic theory of gases, gas molecules are constantly moving in all directions. When they collide with each other and with the walls of the container, they exert pressure. On increasing the pressure on a gas, the gas molecules get closer together. Due to this the space available for the movement of the gas molecules is decreased hence the volume of the gas is reduced. On the other hand, when the pressure on the gas is decreased, gas molecules will move away from each other hence they will have more space to move. Thus, the volume of the gas will be increased.
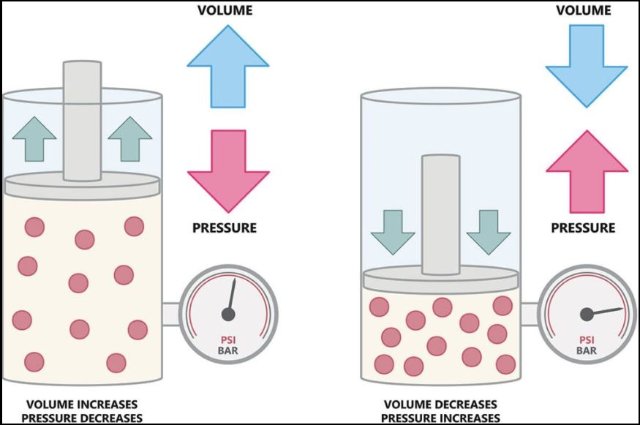
Understanding Boyle’s law with the help of a simple analogy
Suppose a big room is full of people and if the same number of people are placed in a small room they will have to get closer together to fit into the space available in the smaller room. Similarly, when you increase the pressure on a gas, you are essentially increasing the number of gas molecules in a given space. This forces the gas molecules closer together.
Boyle’s Law: Formula
Mathematically, the Boyle’s law or the pressure-volume relationship can be expressed as follows:
P1V1 = P2V2
Where,
P1 = Initial Pressure of the gas
V1 = Initial Volume of the gas
P2 = Final Pressure of the gas
V2 = Final Volume of the gas
Derivation of Boyle’s Law
According to Boyle’s Law, at constant temperature, the pressure of a fixed amount of gas (i.e., keeping the number of gas molecules, n the same) is inversely proportional to its volume.
P ∝ 1/V ( at constant T and n)
⇒ P = k (1/V)
Where, k is the proportionality constant
Thus, PV = k
Therefore, for initial pressure P1 and initial volume V1, we have
P1V1 = k …………..(i)
And, after applying pressure on gas keeping the temperature and amount of gas unchanged, we have
P2V2 = k …………..(ii)
Thus, from equations (i) and (ii), we get
P1V1 = P2V2
Boyle’s Law: Real-world Applications
Boyle's Law has numerous practical applications across various fields some of which are mentioned below:
1. Human Breathing: When you breathe in, your lungs expand. Thus, the volume inside the lungs increases and the pressure inside decreases which as per Boyle’s law. As the concentration of pressure is lower inside the body, the air moves inside the lungs from outside.
2. Scuba Diving: Divers carry compressed air tanks, in which the volume of the gas decreases as the diver descends in water. As a diver ascends, water pressure decreases (following Boyle’s law), so that the air in the tank and body expands to occupy a greater volume. Thus, divers are instructed to carefully manage their ascent to avoid harmful effects due to the expansion of gases as pressure decreases.
3. Medical Ventilation: Mechanical ventilators in hospitals rely on Boyle's Law. The pressure and volume changes created by these control the airflow into and out of the lungs of the patients helping them breathe.
4. Aerospace Engineering: Pressure-volume relationship of gases plays a significant role during the movement of a spacecraft between different atmospheric pressures in space. Thus, Boyle's Law plays a crucial role in designing spacecraft and life support systems for astronauts.
5. Syringe: The syringe, used to inject medication into the body is a perfect example of Boyle’s law. When you pull back the plunger of a syringe, the volume of the syringe increases and the pressure inside it decreases helping in loading the medicine into it. On the other hand, when you push the plunger in, the volume of the syringe decreases with an increase in the pressure inside it due to which the medicine comes out and can be injected into the patient’s body.
Limitations of Boyle’s Law
- Boyle’s law is applicable only to ideal gases.
- The law holds true only under specific conditions like constant temperature and amount of gas. If either of these variables changes, the law's accuracy may diminish.
- Additionally, The law holds good only at high temperatures and low pressures. The law fails at high pressures.
Boyle's Law is a fundamental principle that helps us understand the behaviour of gases in relation to changes in pressure and volume. The law can be used by scientists to further create technological advancements that impact our world.
Also Read: Kohlrausch’s Law: Definition, Formula and Applications, Download PDF
Comments
All Comments (0)
Join the conversation