CBSE Class 10 Board Exam 2021-22: The Central Board of Secondary Education, CBSE has yet again brought an excellent practice material for class 10 students who will take their first MCQ type exam this year. The Board has released additional practice questions for Class 10 Science. There are total 60 questions in this practice set. These are the multiple choice type questions from the whole term 1 syllabus of class 10 Science. Answers to all questions are written at the end. All the CBSE Class 10 Science Extra Practice Questions for Term 1 Exam 2021-22 can be downloaded from here in PDF and saved for last minute revision.
Check the questions and answers below:
1. Some reactions require conditions like specific temperature, pressure, etc. While writing chemical equations for such reactions, where are these conditions usually mentioned?
A. above the arrow
B. along with products
C. below the plus signs
D. before the reactants
Answer: A. above the arrow
2. Given here is the equation of a chemical reaction.
magnesium + oxygen ----------> magnesium oxide
Which of the following can be said about the equation?
A. Only the products are written on the left side of the equation.
B. Only the reactants are written on the left side of the equation.
C. Both the reactants and the products are written on the left side of the equation.
D. Both the reactants and the products are written on the right side of the equation
Answer: B. Only the reactants are written on the left side of the equation.
Also Read: CBSE Class 10 Science Case-study Questions for Term 1 Exam 2021
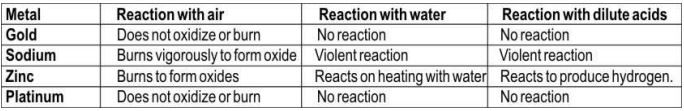
3. Listed here is the reactivity of certain metals.
Which of the above metals are likely to be obtained in their pure states from the Earth's crust?
A. gold only
B. sodium only
C. gold and platinum
D. zinc and sodium
Answer: C. gold and platinum
4. Chemical equations are balanced to reflect that ___________________.
A. matter can change its state during chemical reactions
B. matter cannot be created or destroyed during chemical reactions
C. heat is an important input in chemical reactions
D. all chemical reactions are always reversible
Answer: B. matter cannot be created or destroyed during chemical reactions
5. Which of the following reactions is a neutralisation reaction?
A. 4Na + O2 ---> 2 Na2O
B. Fe + 2HCl ---> FeCl2 + H2
C. MgO + H2O ---> Mg(OH)2
D. HNO3 + NaOH ---> NaNO3 + H2O
Answer: D. HNO3 + NaOH ---> NaNO3 + H2O
6. Which of the following is TRUE about a combination reaction?
A. The number of reactants is always greater than the number of products.
B. The number of products is always greater than the number of reactants.
C. The number of products is always equal to the number of reactants.
D. (Any of the above can be true for different reactions.)
Answer: A. The number of reactants is always greater than the number of products.
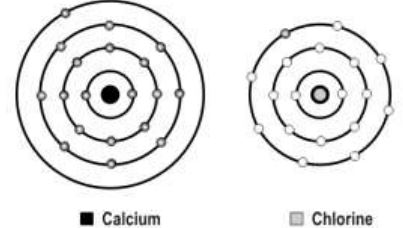
7. A scientist is attempting to represent an ionic bond between calcium and chlorine. The figure below shows the progress he has made so far.
What should be the next step in his representation of the ionic bond?
A. Transfer an electron from the calcium atom to the chlorine atom.
B. Transfer an electron from the chlorine atom to the calcium atom.
C. Add another chlorine atom to accept an electron from the calcium atom.
D. Add another calcium atom to donate an electron to the chlorine atom.
Answer: C. Add another chlorine atom to accept an electron from the calcium atom.
8. In which of the following forms do electrovalent compounds conduct electricity?
A. only in solid form
B. both in solid form and in aqueous solution
C. both in aqueous solution and in molten form
D. in solid form, molten form and in aqueous solution
Answer: C. both in aqueous solution and in molten form
9. Sodium comes after potassium in the reactivity series, so sodium is ___________________.
A. not reactive
B. more reactive than potassium
C. equally reactive as potassium
D. less reactive than potassium
Answer: D. less reactive than potassium
Also Read: CBSE Class 10 Science Chapter-wise MCQs with Answers
10.Which of the following are properties of acids?
P. They are bitter in taste.
Q. They react with metals to produce hydrogen gas.
R. They are easily soluble in water.
A. only P
B. only P and R
C. only Q and R
D. all - P, Q and R
Answer: C. only Q and R
11. Organisms break down large food molecules to small molecules. How does this breakdown help the organisms?
A. It releases a lot of energy in the digestive tract that can be used up by the cells.
B. It ensures that there are enough raw materials to produce and supply oxygen to the cells.
C. It converts the large molecules to small molecules that can pass through the cell membrane.
D. It makes sure that the liberation of heat by the breakdown of large molecules does not occur inside the cell.
Answer: C. It converts the large molecules to small molecules that can pass through the cell membrane.
12. The liver secretes bile, needed to digest fats in our food. The pancreas secretes several enzymes needed to break down food. Which of the following is true of the food that we eat?
A. It passes only through our liver.
B. It passes only through our pancreas.
C. It passes through both our liver and pancreas.
D. It passes neither through our liver nor pancreas.
Answer: D. It passes neither through our liver nor pancreas.
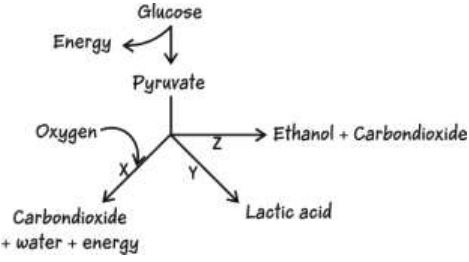
13. Which of the following occurs during oxygen shortage in muscle cells?
A. only X
B. only Y
C. only Z
D. any of them - X, Y or Z
Answer: B. only Y
14. Which of the following plays the important role of creating a suction force which pulls water upwards from the roots of a tree to its leaves?
A. gravitation
B. respiration
C. transpiration
D. photosynthesis
Answer: C. transpiration
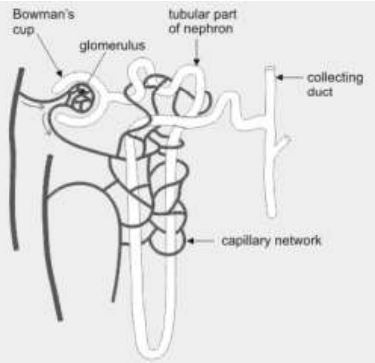
15. Observe the image of a single nephron.
The amount of liquid passing through in the form of glomerular filtrate is approximately 150 - 180 litres per day whereas the amount of urine flowing out of all the nephrons is only 1.5 to 1.8 litres per day.
Water is getting reabsorbed.
In which part of the nephron could the water be getting reabsorbed?
A. in the Bowman's cup
B. in the long tubular part
C. in the collecting duct
D. in the glomerulus
Answer: B. in the long tubular part
All 60 questions can be downloaded in PDF from the link provided below:
| CBSE Class 10 Science Additional Practice Questions for Term 1 Exam |
Important* CBSE Class 10 Science Most Important Resources for Term 1 Exam Preparations
Comments
All Comments (0)
Join the conversation