Water is one of the most well-known compounds, which is made up of two elements, Hydrogen and Oxygen, on the Earth, although it is still surprisingly misconceived. We consume it daily, watch it run along rivers and sleep in lakes and extend endlessly across the oceans. Since we tend to say that water is clear, in this case, its colour varies significantly with depth, light, and the surroundings that it traverses. Crystal clear springs, as dark blue as seas, dark brown as murk, can all attest to the story behind the water. Knowing the colour of water is not only a curiosity, but it also assists us in reading telling signs about the well-being of the ecosystem, water quality, as well as the influence of the natural and human surroundings.
What is the Colour of Water?

Source: shutterstock
Water is not pure in its pure form of scientific use; it is not completely colourless. In pure water, a slight tibation of blue colour is visible and can only be seen when the water is passed by a lot of light, as is the case in oceans, deep lakes or large swimming pools. This blue colour is too minute in small amounts (such as a glass of water) that our human eye does not notice, and water appears to have no colour.
Why Does Water Appear Blue?

Source: shutterstock
The water has a blue colour due to the effect of sunlight on it. Once filming on water, the water molecules absorb the light of the red spectrum of the visible range more intensely. The blue wavelengths are least absorbed, hence they travel further, and they are reflected to the eyes.
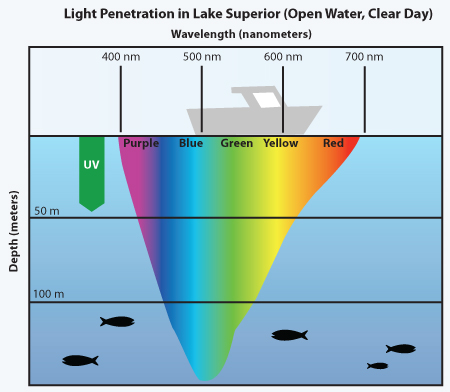
Source: manoa.hawaii.edu
Depth plays a major role:
-
Deep water has a blue colour due to the increased light absorption of red on its descent down the ray of sunshine.
-
Shallow water seems to be of a clear colour since light does not penetrate through a large amount of water to indicate the blue colour.
-
The pools, even indoors, look clear and blue, which proves that it is the water itself rather than the reflection of the sky.
Colours of Water and What They Reveal About Ecosystems
Water is seldom clear in the natural habitats. Various colours offer important evidence concerning the contents in water and the state of the environment.

Source: Shutterstock
Green
-
It is a result of algae that contain chlorophyll.
-
Usually common in nutrient-rich water or stagnant water.
-
May cause signal eutrophication of excess nutrients.
Brown or Yellow
-
Result of dissolved organic matter or soil runoff
-
Often seen after heavy rains or in forested regions
-
Indicates decaying leaves, plant material, or erosion
Red or Rusty Brown
-
Caused by iron-rich minerals or iron bacteria
-
Common in groundwater and areas with iron-heavy soils
Cloudy or Milky White
-
Due to suspended fine particles or air bubbles
-
Can result from erosion, construction, or disturbed sediments
Black
-
Indicates high levels of dissolved organic matter
-
Often linked to polluted or oxygen-poor water
Pink or Reddish-Pink
-
Caused by specific algae or bacteria producing pigments
-
Seen in rare environments like salt-rich lakes
Blue-Green
-
Often linked to cyanobacteria (blue-green algae)
-
Can release toxins harmful to aquatic life and humans
Why it is Important to learn about water colour?
Water colour is one of the natural markers of environmental well-being. Colour transitions may indicate pollution, algal growth, depletion of oxygen or soil erosion. To communities, the knowledge aids in locating safe sources of water as well as conserving ecosystems. In conservation and land management plans, water colour observation enhances sustainable planning, water management and its long-term environmental protection.
Conclusion
Even though water can be seen as clear, it inherently possesses a faint blue colour and reflects the environment with different colours. Such visual changes can give crucial information on the quality of water, the health of the ecosystem, and human influence. Having the ability to read the colour of water means that we get to understand better the protection, safeguarding, and sustainable management of this vital natural resource.
Comments
All Comments (0)
Join the conversation