Do you think soap and detergent are the same thing? Think again! Soap and detergent are both used as cleaning agents, but their compositions and applications differ. Let's first learn exactly what soaps and detergents are.
What are soaps?
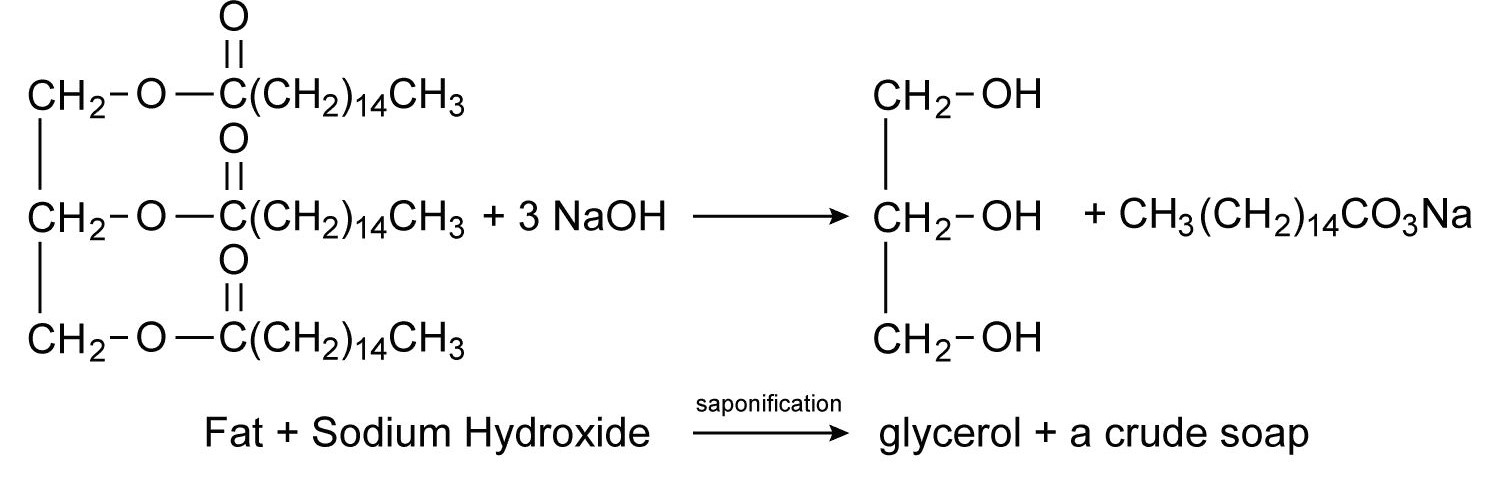
Soaps are cleaning agents that are made from animal fats and vegetable oils. To manufacture soap, glyceryl esters of fatty acids or fats are heated with an aqueous NaOH solution. This is a process known as saponification, which results in the formation of glycerol as a by-product.
Also Read | What Is The Difference Between Reversible and Irreversible Reaction?
What are detergents?
Detergents are cleaning agents made from synthetic surfactants that are used to remove dirt and stains from surfaces, fabrics, and other materials. To manufacture synthetic detergents, petroleum chemicals such as lauryl alcohol are combined with sulphuric acid (H2SO4) and converted into sodium salt.

Also Read | What Is The Difference Between Heart Attack And Cardiac Arrest?
Here are the differences between soap and detergents:
| Soap | Detergent |
| Soaps are made from natural ingredients like animal fat and plant oils, which are biodegradable. | Detergents are usually synthesized in a laboratory using synthetic ingredients, which are not as biodegradable as natural ingredients |
| Cleaning soaps are sodium or potassium salts of long chain fatty acids such as stearic, oleic, and palmitic acids. | Detergents are long chain carboxylic acids of ammonium or solphonate salts. |
| A '-COONa' group is connected to a fatty acid with a long alkyl chain. | A '-SO3Na' group is connected to a lengthy alkyl chain. |
| They are easily biodegradable. | They are not easily biodegradable. |
| They are not effective in hard water and produce scum. | They do not lose their effectiveness in hard water. |
| Examples include sodium palmitate, sodium stearate, and sodium oleate. | Examples include deoxycholic acid and sodium lauryl sulfate. |
How do soaps and detergents clean?
Soap molecules have a polar salt on one end that is hydrophilic in nature (attracted to water). The molecule's opposite end is a nonpolar chain of fatty acids or hydrocarbons that is hydrophobic in nature (repels water but attracts grease and other greasy things).
Let's take the example of washing your hands. When you mix soap with water and then rub your hands together, the grease molecules on your hands dissolve into the soap molecule as its hydrophobic end attracts them. Then, the hydrophilic end of the soap molecule helps to suspend the grease molecules in water, making it easier to rinse them off.
Once the greasy grime and germs have been removed from your hands, the soap molecules completely surround them and create tiny clusters known as micelles that prevent them from sticking to anything else as they wash down the drain.
To sum it up, the difference between soap and detergent lies in the types of molecules each contains and how each react in different conditions.
Also Read | What Is The Difference Between Reflex Action And Walking?
What Is The Difference Between Weather And Climate?
Comments
All Comments (0)
Join the conversation