CBSE Class 12 Chemistry Chapter 4 Competency-Based Questions With Answer Key 2024-25: Assessment is important in education as it reinforces the knowledge that the students gain throughout the course. These questions are designed to evaluate how well the students understand a particular subject or topic.
For the academic year 2024-25, CBSE has released competency-based questions with the answer key that can help the students to prepare well for the exam. Students who want to excel in their exams can take a look at these questions for better preparation.
In this article, we will be providing you with the competency-based questions for Class 12 Chemistry Chapter 3 Chemical Kinetics.
CBSE Class 12 Chapter 4 Competency-Based Questions With Answer Key 2024-25
| Q. No | Question | Marks |
| Multiple Choice Question | ||
| Q.80 | The reaction shown below illustrates the Haber process for manufacturing ammonia: In this reaction, molybdenum acts as a promoter, and iron is used as a catalyst. Which of these is TRUE for the reaction? A. Iron changes the activation energy of the reaction and molybdenum increases the efficiency of the catalyst. B. Iron changes the equilibrium constant and molybdenum changes the Gibbs energy of the reaction. C. Iron changes the enthalpy of the reaction and molybdenum changes the equilibrium constant. D. Iron and molybdenum change the Gibbs energy of the reaction. | 1 |
| Q.81 | Which of the following can INCREASE the rate of a chemical reaction? P) Increasing the temperature Q) increasing the concentration of products R) Adding a catalyst S) Increasing the concentration of reactants A. Q and S B. P and Q C. P, R, and S D. All- P, Q, R, and S | 1 |
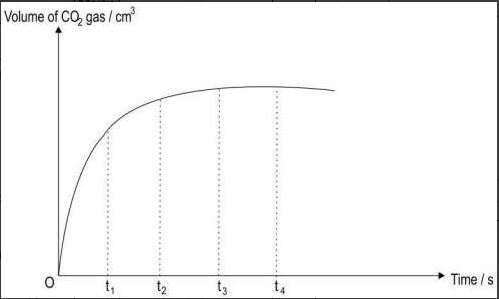
| Q.82 | The graph below shows the volume of carbon dioxide formed with time during a chemical reaction: | 1 |
| Based on the graph, at what time is the rate of reaction the highest? A. t1 B. t2 C. t3 D. t4 | ||
| Q.83 | A living organism takes in different amounts of different isotopes of the same element from the environment. The organism takes these isotopes in the same relative proportion that existed naturally in the environment. For example, it takes carbon-12 and carbon-14 and once the organism dies, it stops replenishing its carbon supply, and the total carbon-14 content in the organism slowly disappears. This is because C-14 is radioactive and it decays as it's less stable whereas C-12 is very stable and does not decay. Scientists can determine how long ago an organism died by measuring how much carbon-14 is left relative to carbon-12. Similarly, the organism's age can also be found by measuring how much potassium-40- 40 or beryllium-10 is present about potassium-39 and beryllium-9. Below is the half-life of: Carbon-14 = 5730 years Potassium-40 = 1.26 billion years Beryllium-10 = 1.52 billion years Based on the above data, which of the following dating methods can be used to determine the exact age of a living organism from its remains which are estimated to be 100,000 years old? | 1 |
| (I) Carbon dating (II) Potassium dating (III) Beryllium dating A. I only B. I and II only C. II and III only D. All- I, II, and III | ||
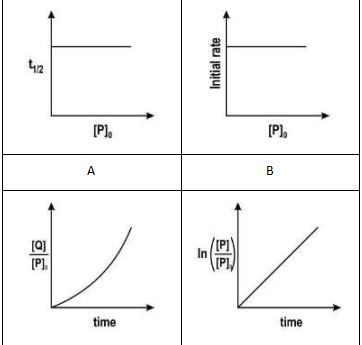
| Q.84 | The hydrolysis of tert-butyl bromide with an aqueous NaOH solution (as shown below) is an example of an SN1 reaction. 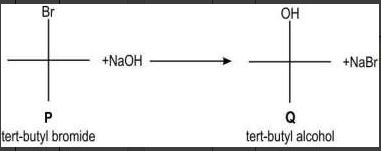 Which of the following graphs correctly represents the above reaction? | 1 |
Answers
| Q. No | Answers | Marks |
| Q.80 | A. Iron changes the activation energy of the reaction and molybdenum increases the efficiency of the catalyst. | 1 |
| Q.81 | C. P, R, and S | 1 |
| Q.82 | A. t1 | 1 |
| Q.83 | C. II and III only | 1 |
| Q.84 | A. | 1 |
| Q.85 | A. P | 1 |
To download the rest of the questions with the answer key, we are providing a FREE PDF that can be downloaded easily.
Other Related Links
Comments
All Comments (0)
Join the conversation