Application Of Henry's Law: Chemistry is an important subject for science students, and its percentage is considered if you plan to be admitted to higher education. There are various formulas and chemical equations in chemistry that are difficult for students to learn and understand. One such topic is Henry’s law. Here we will be discussing Henry's law derivation, Henry's law solubility formula, Henry’s law constant, and application of Henry's law. Along with these, we will show a few examples where you can use Henry’s law. Read these Henry's Law notes.
What is Henry’s Law?
According to the NCERT Class 11 Chemistry Chapter 6 Equilibrium definition, Henry’s law states that the mass of a gas dissolved in a given mass of a solvent at any temperature is proportional to the pressure of the gas above the solvent. According to NCERT Class 12 Chemistry Chapter 1 Solutions, Henry’s law states that at a constant temperature, the solubility of a gas in a liquid is directly proportional to the partial pressure of the gas present above the surface of the liquid or solution.
Both of these definitions are similar and say that when the temperature is constant, the amount or mass of gas dissolved in a solution is directly proportional to the partial pressure of that gas above the liquid.
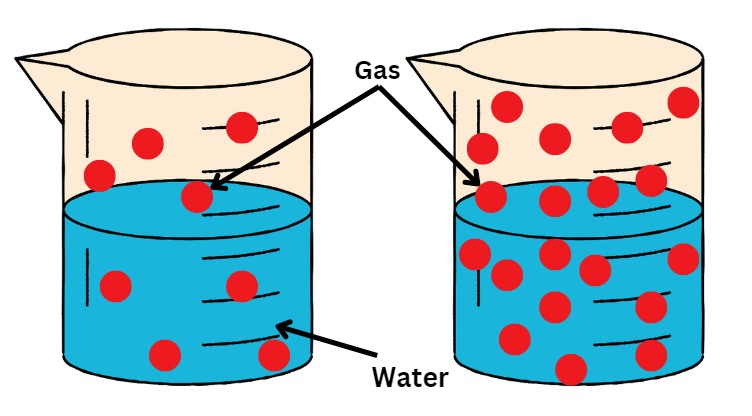
Henry's Law Solubility Formula
If ‘P’ is the pressure and ‘C’ is the gas concentration then
P∝C
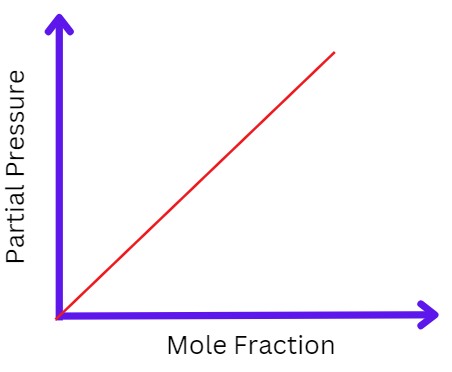
To remove ∝ we can put a constant KH, now the formulas become;
P= KHC
KH is the Henry’s law constant.
As per the NCERT textbook ‘C’ can be replaced with ‘𝓧’ which denotes the mole fraction of the gas. Now the formula can be rearranged as;
P= KH𝓧
Henry’s Law of Constant
Henry’s law constant is denoted by KH and it is affected by:
- Nature of the gas
- Nature of solvent
- Temperature
- Pressure
Because of this, different gasses have different KH.
Applications of Henry’s Law
1. Production of carbonated drinks
In NCERT language ‘The soda water bottle is sealed under pressure of gas when its solubility in water is high. As soon as the bottle is opened, some of the dissolved carbon dioxide gas escapes to reach a new equilibrium condition required for the lower pressure, namely its partial pressure in the atmosphere. This is how the soda water in a bottle when left open to the air for some time, turns ‘flat’.’
It means that in carbonated drinks, the amount of carbon dioxide is higher than normal because the bottles are packaged at a higher pressure than atmospheric pressure. Thus, as soon as we open the bottles, the gas escapes out of the carbonated drink to maintain equilibrium.
2. Maintain blood oxygen levels
In the blood, the oxygen concentration needs to be higher to get into the tissues and cells. Gas molecules across the cell membrane move in the direction from higher partial pressure to lower partial pressure. Thus, when the oxygen gas’s partial pressure is higher in the blood, the oxygen molecule concentrations increase in the cells and tissues, and vice versa.
Limitations of Henry’s Law
1. This law is applicable when the molecules of the system are in a state of equilibrium.
2. The law is not applicable during a reaction between solvent and gas molecules.
3. This law is not applicable when gases are placed under extremely high pressure.
Solved Henry's Law Question Examples
Q1. If N2 gas is bubbled through water at 293 K, how many millimoles of N2 gas would dissolve in 1 litre of water? Assume that N2 exerts a partial pressure of 0.987 bar. Given that Henry’s law constant for N2 at 293 K is 76.48 kbar.
Sol. The solubility of gas is related to the mole fraction in aqueous solution.
The mole fraction of the gas in the solution is calculated by applying Henry’s law. Thus:
x (Nitrogen) = p(nitrogen)/KH = 0.987bar/76,480 bar = 1.29 × 10–5
As 1 litre of water contains 55.5 mol of it, therefore if n represents
number of moles of N2 in solution,
x (Nitrogen) = n mol/n mol + 55.5 mol = n/ 55.5 = 1.29 × 10–5
(n in denominator is neglected as it is < < 55.5)
Thus n = 1.29 × 10–5 × 55.5 mol
= 7.16 × 10–4 mol
= 7.16×10 mol × 1000 mmol/1 mol
= 0.716 mmol
Q2. Find the solubility of gaseous oxygen in water at a temperature of 293 K when the partial pressure exerted by O2 is 1 bar. (Given: kH for O2 34840 bar.L.mol-1)
Sol. Henry’s law P = kH x C
Substituting, kH = 34840 bar.L.mol-1 and P = 1 bar, the equation becomes
C = 1/34840 mol.L-1 = 2.87 x 10-5 mol/L
Thus, the solubility of oxygen in water under the given conditions is 2.87 x 10-5 M.
Also Read:
Comments
All Comments (0)
Join the conversation