AP Inter 2nd Year Chemistry Question Paper 2024: The Board of Intermediate Education Andhra Pradesh (BIEAP) conducted the AP Inter 2nd Year Chemistry paper today, March 15, 2024. The exam was held for a duration of 3 hours from 9:00 AM to 12 noon. Here’s the post exam analysis for the AP Inter 2nd Year Chemistry Paper 2024. Check the reactions of students and experts to know the overall difficulty level of the exam. Also, check the question paper for AP Inter 2nd Year Chemistry 2024 along with its answer key. Students can use this answer key to cross-check their answers and assess their performance in the AP Intermediate 2nd Year Chemistry Exam 2024.
Pattern of AP Inter 2nd Year Chemistry Question Paper 2024
The question paper had a total of 21 questions divided into three sections:
- Section A: No. 1-10 were Very Short Answer Type Questions of TWO marks each.
- Section B: No. 11-18 were Short Answer Type Questions of FOUR marks each.
- Section C: No. 19-21 were Long Answer Type Questions of EIGHT marks each.
All questions in Section A were compulsory while sections B and C had choices for questions.
AP Inter 2nd Year Chemistry Exam Analysis 2024
According to the initial feedback shared by students and subject teachers, the AP Inter 2nd Year Chemistry Paper was of moderate difficulty level. Most questions in the exam paper were direct while 2-3 questions of 4 marks seemed challenging to some students. Section C included easier questions in comparison to Section B. Overall, the paper was perceived as manageable, with most students expecting to score between 70-80% in the exam.
AP Inter 2nd Year Chemistry Question Paper 2024

Download the complete question paper in PDF below:
AP Inter 2nd Year Chemistry Question Paper 2024 PDF |
AP Inter 2nd Year Chemistry Answer Key 2024
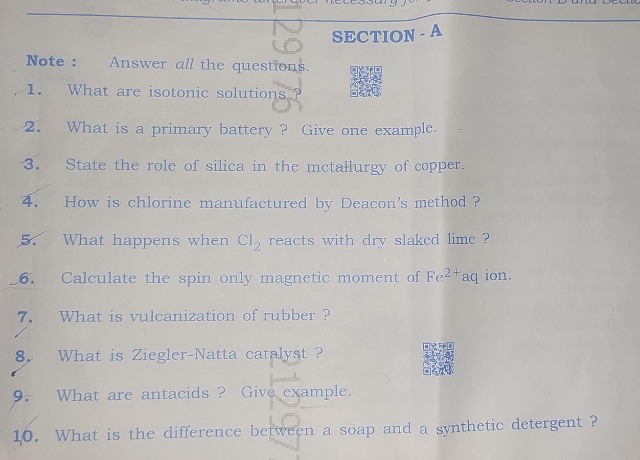
SECTION - A
1.What are isotonic solutions?
Answer: The solutions having the same osmotic pressure at a given temperature are called isotonic solutions. For example, the osmotic pressure associated with fluid inside the blood cell is equivalent to that of 0.9% NaCl solution thus they are isotonic solutions.
2.What is a primary battery? Give one example.
Answer: The batteries which after their use over a period of time, become dead and cannot be recharged, are known as primary batteries. For example: dry cells.
3.State the role of silica in the metallurgy of copper.
Answer: The role of silica in the metallurgy of copper is to remove the iron oxide obtained as slag during the process of extraction of pure copper from copper pyrite.
4.How is chlorine manufactured by Deacon's method?
Answer: In Deacon's process chlorine is obtained by the oxidation of hydrogen chloride gas by atmospheric oxygen in the presence of CuCl2 (catalyst) at 723 K.
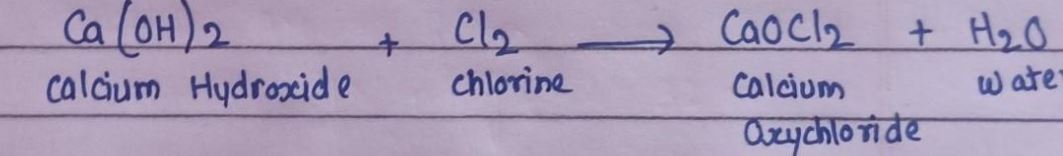
5.What happens when Cl2 reacts with dry slaked lime?
Answer: Chlorine reacts with dry slaked lime to produce water and calcium oxychloride, commonly known as bleaching powder.
6.Calculate the spin only magnetic moment of Fe²+aq ion.
Answer:
Magnetic moment is given by, μ=√[n(n+2)]
In Fe²+, 4 unpaired electrons are present.
Therefore, μ = μ=√[4(4+2)]= √24 BM =4.90 BM
7.What is vulcanization of rubber?
Answer: Vulcanization of rubber is the process of converting natural rubber to a more strong and elastic form. It includes cross-linking rubber molecules chemically with organic/inorganic substance through the action of heat and pressure.
8.What is Ziegler-Natta catalyst?
Answer: Ziegler-Natta catalyst is a mixture of titanium tetrachloride TiCl4 and triethyl aluminium chloride Al(C2H5)3. It is used in the synthesis of polymers of 1-alkenes (alpha-olefins).
9.What are antacids? Give example.
Answer: An antacid is a chemical compound, which neutralizes excessive hydrochloric acid released in the stomach. For example, sodium bicarbonate.
10.What is the difference between a soap and a synthetic detergent?
Answer: Difference between a soap and a synthetic detergent
| Soap | Detergent |
| Soaps are the sodium salts of carboxylic acids in long chains | Detergents are sodium salts of long-chain benzene sulphonic acids |
| Soaps are biodegradable. | Some of the detergents can not be biodegraded |
| Soaps have relatively weak cleaning action. | Detergents have a strong cleaning effect. |
| Soaps cannot be used in hard water. | Detergents can be used in hard water. |
Also Check| AP Inter 1st and 2nd Year Date Sheet 2024
Comments
All Comments (0)
Join the conversation