Hydroxide; Definition, Formula, Reactions And Examples: Hydroxide is a negatively charged ion consisting of one oxygen and one hydrogen atom bound together by a covalent bond.
It is widely used as a food preservative, for the alkalization of urine to prevent kidney stones, and as an anticoagulant for stored blood and buffer.
Here’s a breakdown of the key aspects of Hydroxide:
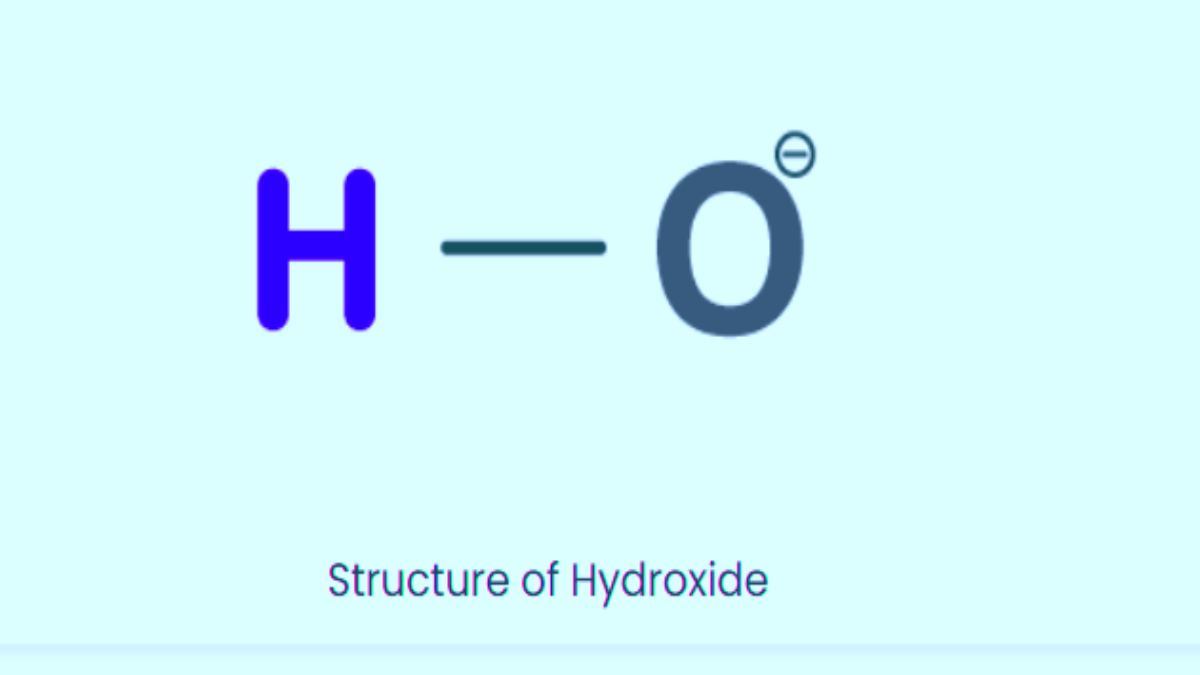
- Structure: The hydroxide ion has a single covalent bond between oxygen and hydrogen, with the oxygen atoms holding the extra electron giving it a negative charge.
- Behaviour: Hydroxide ions act as bases in chemical reactions. They accept protons from acids forming water molecules.
- Importance: Hydroxide ions are fundamental to understanding acid-base chemistry.
Reactions That Involve Hydroxide Ions
Hydroxide ions are versatile participants in many chemical reactions. Have a look at some of the key reactions involving the hydroxide.
- Neutralisation Reactions:
This is the classic acid-base reaction. Here’s a general reaction
Hydrochloric acid (HCl) + Sodium hydroxide (NaOH) -> Sodium chloride (NaCl) + Water (H2O)
- Hydrolysis Reactions:
Certain salts react with water to produce hydroxide ions and other iconic species.
Sodium acetate (NaC2H3O2) + Water (H2O) -> Sodium hydroxide (NaOH) + Acetic acid (HC2H3O2)
- Precipitation Reactions:
When soluble ionic compounds containing metal cations react with hydroxide ions, they can form insoluble precipitates.
Silver nitrate (AgNO3) + Sodium hydroxide (NaOH) -> Silver oxide (Ag2O)↓ + Sodium nitrate (NaNO3)
- Nucleophilic Substitution Reactions:
Hydroxide ions, due to their electron-rich nature, can act as nucleophiles in reactions with organic molecules.
- Amphoteric Hydroxide Reactions:
Some metal hydroxides can react with both acids and bases.
Aluminium hydroxide (Al(OH)3) + Sodium hydroxide (NaOH) -> Sodium aluminate (NaAlO2) + Water (H2O)
| Property | Value |
| Formula | OH- |
| Charge | -1 |
| Size | Small |
| Basicity | Strong Base |
| Solubility in Water | Highly Soluble |
| Monoisotopic mass of Hydroxide | 17.003 g/mol |
| Molecular Weight of Hydroxide | 17.007 g/mol |
Structure Of Hydroxide
Uses Of Hydroxide
- It can be used to produce fuel cells.
- They can be used in processing paper pulp and treating textiles.
- It is a key ingredient in the setting of the cement.
- Used in the extraction of alumina.
- It can also help purify the water by removing impurities and adjusting pH.
Video Lessons For Students
Jagran Josh has a special portal for students where video lessons are provided by the subject matter experts for the learners to excel in their particular subjects.
- CBSE Class 11 Science Video Courses And Mock Tests By Subject Matter Experts
- CBSE Class 12 Science Video Courses And Mock Tests By Subject Matter Experts
Other Related Links
Comments
All Comments (0)
Join the conversation