Think of a cold, refreshing drink on a hot day. Do you see the ice cubes floating? Have you ever wondered why ice cubes float on the surface of a glass of water? The phenomenon of ice floating in water is a direct consequence of the molecular and physical properties of water, which make it one of the most unique substances in nature. While most materials become denser as they transition from liquid to solid, water defies this typical behaviour due to its highly structured hydrogen-bonding network.
Why Does Ice Float On Water?
This anomalous behaviour of ice can be explained by fundamental principles in chemistry and physics, including the molecular geometry of water, density differences, and buoyancy governed by Archimedes' Principle. Let us dive into the science behind it.
The Molecular Structure of Water
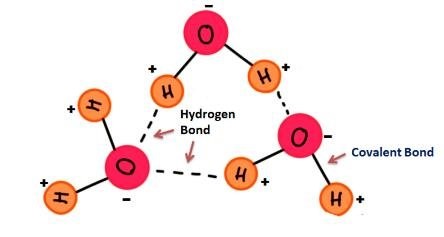
At the core of understanding why ice floats is the unique molecular structure of water. Water (H₂O) consists of two hydrogen atoms and one oxygen atom. These atoms are bonded covalently, forming a bent structure. Due to its bent shape and the differences in electronegativity between hydrogen and oxygen, the water molecule becomes polar. This means that one end (where oxygen is located) carries a partial negative charge, while the hydrogen atoms carry partial positive charges.

This polarity causes water molecules to attract each other through hydrogen bonds, where the positive hydrogen atoms of one water molecule interact with the negative oxygen atoms of another. These bonds are constantly forming and breaking as water molecules move, particularly in liquid form. This interaction among molecules is a key reason behind water's unique properties, such as its high boiling point and its ability to dissolve many substances.
Science Quiz On Solar Eclipse With Answers
Water’s Behavior as It Freezes
As the temperature of water decreases, so does the kinetic energy of its molecules. When water cools to 0°C (32°F), it begins to freeze. During this phase change, the water molecules move more slowly, and the hydrogen bonds between them become more stable. This results in the formation of a crystalline structure known as ice.
In ice, each water molecule is bonded to four other water molecules, creating a lattice. This lattice arrangement is more spread out than in liquid water, meaning the molecules in ice are further apart. The increased spacing results in a lower density compared to liquid water. While most substances become denser as they freeze, water is an exception due to this open lattice structure.
The Concept of Density
Density is a fundamental concept in understanding why ice floats. Density is defined as mass per unit volume. For liquid water, the density is approximately 1.00 grams per cubic centimetre (g/cm³) at 4°C, which is its densest point. Ice, on the other hand, has a density of about 0.92 g/cm³, meaning it is less dense than liquid water.
Since density is directly related to whether an object will sink or float, this lower density of ice compared to water explains why it floats. If ice were denser than liquid water, it would sink, just as most other solids sink in their respective liquids.
Buoyancy and Archimedes’ Principle
To understand why ice floats, we also need to consider buoyancy, a key principle in physics. Archimedes' Principle states that an object submerged in a fluid experiences an upward buoyant force equal to the weight of the fluid it displaces. Whether an object sinks or floats depends on its density relative to the fluid.
When ice is placed in water, it displaces a certain amount of liquid. Since the ice is less dense than the water it displaces, the upward buoyant force is greater than the gravitational force pulling the ice down. As a result, ice floats. Only about 10% of an ice block remains above the water's surface, while the remaining 90% is submerged, as seen with icebergs and ice cubes.
Also read: What is Space Junk (Debris) and why is it a global threat?
The Thermal Properties of Water and Ice
Another factor that plays a role in ice floating is the thermal properties of water, particularly the latent heat of fusion. When water freezes, it releases heat into the environment, a process known as the latent heat of fusion. It takes considerable energy to freeze water because the hydrogen bonds between water molecules must reorganize into a more rigid ice lattice.
Similarly, when ice melts, it absorbs a significant amount of heat from its surroundings. This process explains why ice has such a cooling effect when added to a drink—it absorbs heat as it transitions back into liquid water. These thermal properties also contribute to water's stability in different states and support life by regulating temperatures in natural environments.
Water’s Maximum Density at 4°C
One of the unusual properties of water is that it reaches its maximum density at 4°C (39°F), just before it begins to freeze. As water cools below this temperature, it expands rather than contracts. This expansion happens because the water molecules begin to form the open, hexagonal lattice structure of ice. By the time water freezes, it is 9% less dense than when it was a liquid. This expansion as water transitions into ice is a rare and remarkable phenomenon that explains why ice forms on the surface of lakes rather than sinking.
Also read: Why Are There Stones On Railway Tracks?
Environmental Significance of Ice Floating
The fact that ice floats has crucial implications for the environment, particularly in aquatic ecosystems and polar regions. When ice forms on the surface of bodies of water such as lakes or oceans, it acts as an insulating layer. This layer prevents the colder air above from further cooling the water beneath, allowing aquatic life to survive even in freezing temperatures.
In polar regions, floating sea ice reflects sunlight, helping to regulate the Earth’s temperature by maintaining a cooler climate. This reflective quality, known as the albedo effect, plays a vital role in mitigating global warming. If ice were to sink, it could drastically alter ocean circulation patterns and affect global climate systems.
Everyday Observations and Practical Applications
We witness the effects of ice's buoyancy in everyday life, such as when ice cubes float in a glass of water. The same principles that allow ice to float are also applied in engineering, particularly in the design of ships and floating platforms. These structures rely on the concept of buoyancy to remain stable and afloat.
Furthermore, the density and buoyancy principles behind ice floating have been crucial in scientific research, particularly in understanding climate change, studying glaciers, and exploring ice’s role in maintaining Earth’s climate balance.
Conclusion
The reason ice floats on water can be traced back to the molecular structure of water and the physical principles of density and buoyancy. The hydrogen bonding in water molecules forms a less dense crystalline lattice in ice, allowing it to float on liquid water. This unique property of water has significant environmental implications, particularly in regulating temperatures and preserving aquatic ecosystems.
Comments
All Comments (0)
Join the conversation