Carboxylic Acid: The carbonyl compounds in which carbon of carbonyl group is bonded to carbon or hydrogen and oxygen of hydroxyl moiety (-OH) are known as carboxylic acids. These acids play an important role in biochemical processes of life. They add fragrance and flavour to nature, for example, vanillin (from vanilla beans) and cinnamaldehyde (from cinnamon) have pleasant fragrances.
What are Carboxylic Acids?
Carbon compounds containing a carboxyl functional group, –COOH are called carboxylic acids. It is a combination of carbonyl and hydroxyl. These acids may be aliphatic (RCOOH) or aromatic (ArCOOH) depending on the group.
Also Check: CBSE Science Video Courses For Class 12 Students
Nomenclature of Carboxylic Acids
These acids are usually known by their common names which end with the suffix –ic acid. The guidelines that are followed in the IUPAC nomenclature of carboxylic acids are as follows:
Aliphatic carboxylic acids are named by replacing the ending –e in the name of the corresponding alkane with – oic acid.
In numbering the carbon chain, the carboxylic carbon is numbered one.
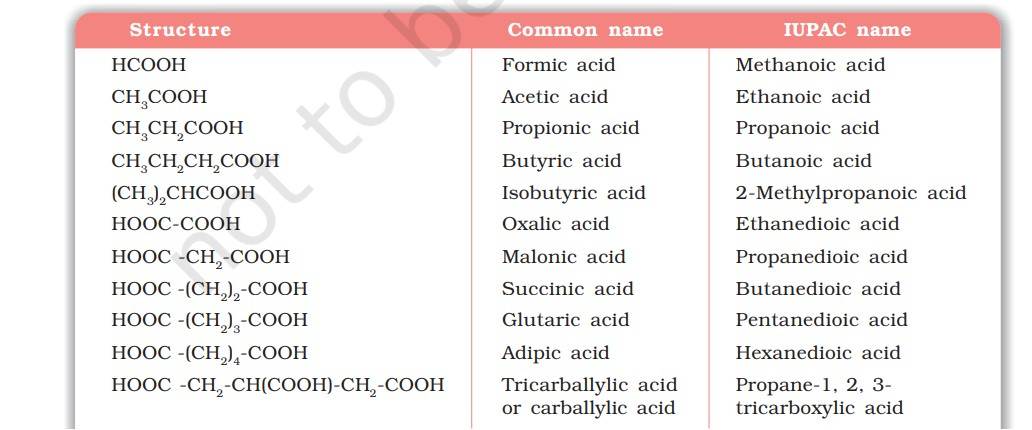
For naming compounds containing more than one carboxyl group, the alkyl chain leaving carboxyl groups is numbered and the number of carboxyl groups is indicated by adding the multiplicative prefix, dicarboxylic acid, tricarboxylic acid, etc. to the name of parent alkyl chain.
The position of –COOH groups are indicated by the arabic numeral before the multiplicative prefix.
Carboxylic Acids Structure
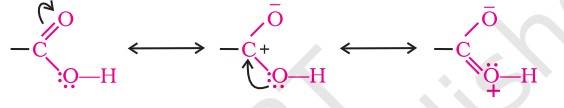
NCERT has explained that, ‘’In carboxylic acids, the bonds to the carboxyl carbon lie in one plane and are separated by about 120°. The carboxylic carbon is less electrophilic than carbonyl carbon because of the possible resonance structure as below.’’
Physical Properties of Carboxylic Acids (Courtesy NCERT)
Aliphatic carboxylic acids upto nine carbon atoms are colourless liquids at room temperature with unpleasant odours.
The higher acids are wax like solids and are practically odourless due to their low volatility.
Carboxylic acids are higher boiling liquids than aldehydes, ketones and even alcohols of comparable molecular masses.
Most carboxylic acids exist as dimer in the vapour phase or in the aprotic solvents.
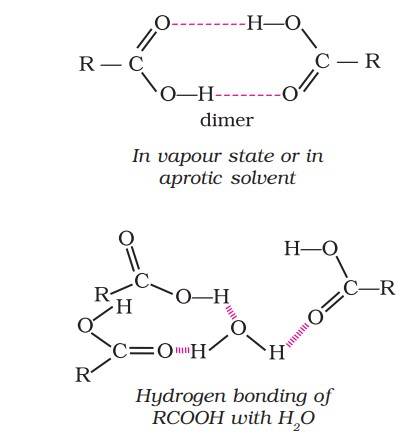
Simple aliphatic carboxylic acids having upto four carbon atoms are miscible in water due to the formation of hydrogen bonds with water.
Higher carboxylic acids are practically insoluble in water due to the increased hydrophobic interaction of hydrocarbon part.
Carboxylic acids are also soluble in less polar organic solvents like benzene, ether, alcohol, chloroform, etc.
Note: All images have been taken from NCERT Class 12 Chemistry Textbook.
Also, check
CBSE Class 12th Chemistry Notes: Aldehydes, Ketones and Carboxylic Acids (Part - III)
CBSE Class 12th Chemistry Notes: Aldehydes, Ketones and Carboxylic Acids (Part - I)
Comments
All Comments (0)
Join the conversation