Molecular Geometry: If you are looking for the topic of molecular geometry, well you have come to the right place. Molecular geometry is an important topic for students. If students understand it well and keep the concepts clear in their mind, they can score well in this area. You can find more explainers in the school section of Jagran Josh. Let us begin by understanding what molecular geometry is.
What is Molecular Geometry?
Molecular geometry can be simply defined as the three-dimensional arrangement of atoms within a molecule. By studying molecular geometry, students can find out a molecule’s shape, bond angles and lengths among other things. Students should understand the structure of a molecule as various properties of a substance are usually determined by its geometry.
Also Check: CBSE Science Video Courses for Class 12 Students
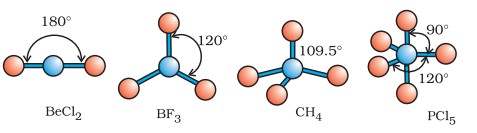
| Types of Molecular Geometry Trigonal planar- In trigonal planar molecular geometry, the center atom has three molecules connected to it. Bond angle is 120 degrees. Tetrahedral- In tetrahedral molecular geometry, there are four bonds connected to a single atom. Bond angle is 109.5 degrees. Linear- In linear molecular geometry, the center atom has two molecules connected to it. Bond angle is 180 degrees. Trigonal-bipyramidal- In this type of molecular geometry, the center atom is surrounded by five atoms. Bond angles are 90 and 120 degrees. Octahedral- In octahedral molecular geometry, there are six atoms around the center atom. Bond angle is 90 degrees. |
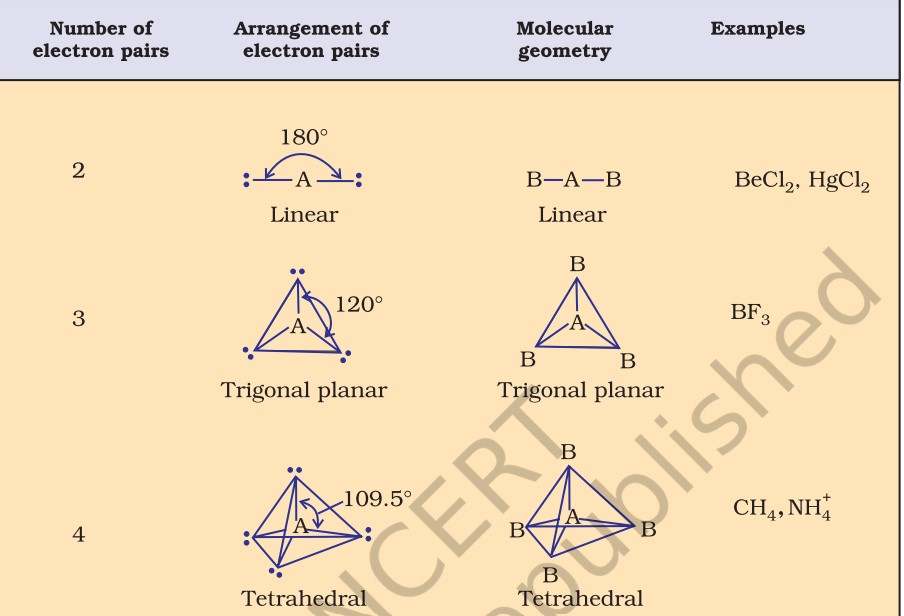
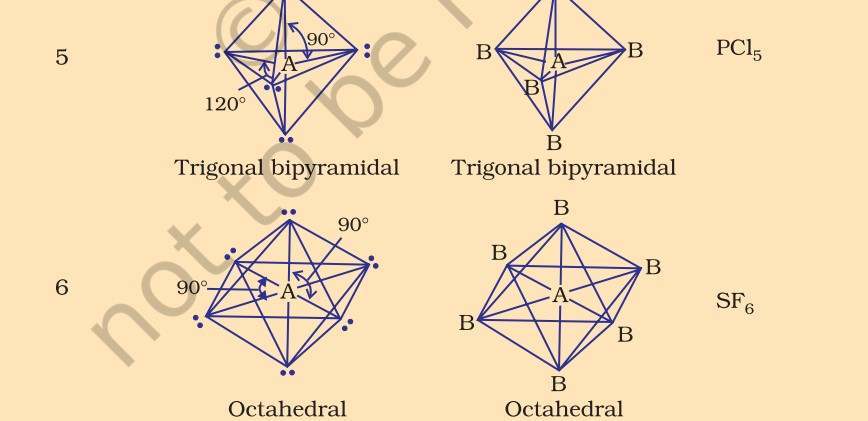
Several methods have been introduced to determine molecular geometry. Some of them are- Kössel-Lewis approach, Valence Shell Electron Pair Repulsion (VSEPR) Theory, Valence Bond (VB) Theory and Molecular Orbital (MO) Theory. Let us take a look at the VSEPR theory.
Valence Shell Electron Pair Repulsion (VSEPR) Theory
NCERT has effectively explained the theory as, ‘’For the prediction of geometrical shapes of molecules with the help of VSEPR theory, it is convenient to divide molecules into two categories as (i) molecules in which the central atom has no lone pair and (ii) molecules in which the central atom has one or more lone pairs. While the lone pairs are localised on the central atom, each bonded pair is shared between two atoms. As a result, the lone pair electrons in a molecule occupy more space as compared to the bonding pairs of electrons. This results in greater repulsion between lone pairs of electrons as compared to the lone pair - bond pair and bond pair - bond pair repulsions. These repulsion effects result in deviations from idealised shapes and alterations in bond angles in molecules. The VSEPR Theory is able to predict geometry of a large number of molecules, especially the compounds of p-block elements accurately. It is also quite successful in determining the geometry quite-accurately even when the energy difference between possible structures is very small.’’
Postulates of VSEPR Theory are-
The shape of a molecule depends upon the number of valence shell electron pairs (bonded or nonbonded) around the central atom.
Pairs of electrons in the valence shell repel one another since their electron clouds are negatively charged.
These pairs of electrons tend to occupy such positions in space that minimise repulsion and thus maximise distance between them.
The valence shell is taken as a sphere with the electron pairs localising on the spherical surface at maximum distance from one another.
A multiple bond is treated as if it is a single electron pair and the two or three electron pairs of a multiple bond are treated as a single super pair.
Where two or more resonance structures can represent a molecule, the VSEPR model is applicable to any such structure.
Note: All images have been taken from NCERT Class 11 Chemistry textbook.
Also, check
MCQs for CBSE Class 11 Chemical Bonding and Molecular Structure: Based on Revised Syllabus
Benzene: Chemical Formula and Molecular Structure of Benzene
Comments
All Comments (0)
Join the conversation