NCERT Class 10 Science Chapter 3 Intext Question Answer: NCERT textbooks are the essential and most important study resources for students. Many national and state-level boards follow its content to educate students. Here, the question-wise solutions are provided for NCERT Class 10 Science Chapter 3, which is about metals and non-metals. The solutions to the intext and chapter-end questions are given below and can be read or downloaded in PDF. Scroll down and find your answers.
NCERT Class 10 Metals and Non-Metals Solutions
The list of solutions for all the questions of this chapter starts from below. Check the answers and download the PDF for further use.
NCERT Class 10 Science Chapter 3 Intext Question Answer Page Number 40
- Give an example of a metal which
(i) is a liquid at room temperature.
(ii) can be easily cut with a knife.
(iii) is the best conductor of heat.
(iv) is a poor conductor of heat.
Answer:
(i) Mercury: It is a metal that remains in liquid form at room temperature.
(ii) Sodium: This metal is soft and can easily be cut with a knife.
(iii) Silver: It is the best conductor of heat among metals.
(iv) Lead: It is a metal that is a poor conductor of heat compared to others.
- Explain the meanings of malleable and ductile.
Answer:
- Malleable: A material is malleable if it can be hammered or pressed into thin sheets without breaking. For example, gold is highly malleable.
- Ductile: A material is ductile if it can be stretched into thin wires. Copper is an example of a highly ductile metal.
NCERT Class 10 Science Chapter 3 Intext Question Answer Page Number 46
- Why is sodium kept immersed in kerosene oil?
Answer: Sodium is a highly reactive metal, especially when it comes into contact with air or water. If exposed to air, it reacts with the moisture and oxygen, forming sodium hydroxide and sodium oxide, which can be dangerous. Sodium can also react violently with water, producing heat and hydrogen gas, which could lead to explosions. To prevent these reactions, sodium is kept immersed in kerosene oil, which acts as a protective layer and prevents it from reacting with air or moisture.
- Write equations for the reactions of
(i) iron with steam
(ii) calcium and potassium with water
Answer: (i) Iron with steam:
When iron reacts with steam, it forms iron oxide and hydrogen gas.
Equation:
3Fe+4H2O (steam)→Fe3O4+4H2
(ii) Calcium with water:
Calcium reacts with water to produce calcium hydroxide and hydrogen gas.
Equation:
Ca+2H2O→Ca(OH)2+H2
(iii) Potassium with water:
Potassium reacts vigorously with water, forming potassium hydroxide and hydrogen gas, with a lot of heat.
Equation:
2K+2H2O→2KOH+H2
- Samples of four metals A, B, C and D were taken and added to the following solution one by one. The results obtained have been tabulated as follows.
| Metal | Iron (II) Sulphate | Copper (II) Sulphate | Zinc Sulphate | Silver nitrate |
| A B C D | No reaction Displacement No reaction No reaction | Displacement No reaction No reaction | No reaction No reaction No reaction | Displacement No reaction |
Use the Table above to answer the following questions about metals
A, B, C and D.
(i) Which is the most reactive metal?
(ii) What would you observe if B is added to a solution of Copper(II) sulphate?
(iii) Arrange the metals A, B, C and D in the order of decreasing reactivity.
Answer: Most reactive metal:
(i) B is the most reactive metal. This is because it displaces copper from copper(II) sulphate solution, which indicates a higher reactivity than copper.
(ii) Observation when B is added to copper(II) sulphate: When metal B is added to copper(II) sulphate, a displacement reaction occurs. Copper will be displaced from the solution, and metal B will form B sulphate, while copper gets deposited.
(iii) Order of decreasing reactivity: Based on the displacement reactions observed, the metals in order of decreasing reactivity are:
B > A > C > D.
This order is deduced from their ability to displace other metals or not. B is the most reactive as it displaces copper, while D is the least reactive as it does not show any displacement reactions.
- Which gas is produced when dilute hydrochloric acid is added to a reactive metal? Write the chemical reaction when iron reacts with dilute H2SO4.
Answer: When dilute hydrochloric acid (HCl) is added to a reactive metal, hydrogen gas (H₂) is produced. This is a common reaction where the acid reacts with the metal, displacing hydrogen from the acid.
Chemical reaction of iron with dilute sulphuric acid (H₂SO₄): Fe+H2SO4→FeSO4+H2
- What would you observe when zinc is added to a solution of iron(II) sulphate? Write the chemical reaction that takes place.
Answer: When zinc is added to a solution of iron(II) sulphate (FeSO₄), a displacement reaction occurs. Zinc is more reactive than iron, so it displaces iron from the iron(II) sulphate solution. You would observe that iron is deposited as a solid, and the solution's colour might change because iron(II) sulphate is light green, while the newly formed zinc sulphate (ZnSO₄) is colourless.
The chemical reaction is:
Zn+FeSO4→ZnSO4+Fe
In this reaction, zinc replaces iron in the compound, forming zinc sulphate and iron as a product.
NCERT Class 10 Science Chapter 3 Intext Question Answer Page Number 49
- (i) Write the electron-dot structures for sodium, oxygen and magnesium.
(ii) Show the formation of Na2O and MgO by the transfer of electrons.
(iii) What are the ions present in these compounds?
Answer: (i) 
(ii) 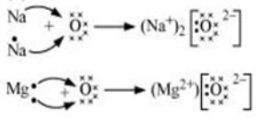
(iii) Na₂O: The ions present are Na⁺ (sodium ion) and O²⁻ (oxide ion).
MgO: The ions present are Mg²⁺ (magnesium ion) and O²⁻ (oxide ion).
- Why do ionic compounds have high melting points?
Answer:
Ionic compounds have high melting points because they are made up of a lattice structure of positively and negatively charged ions held together by strong electrostatic forces of attraction. These forces, known as ionic bonds, are very strong and require a lot of energy to break. This is why ionic compounds like Na₂O and MgO typically have high melting and boiling points.
NCERT Class 10 Science Chapter 3 Intext Question Answer Page Number 53
- Define the following terms.
(i) Mineral (ii) Ore (iii) Gangue
Answer: (i) Mineral: A mineral is a naturally occurring substance, usually solid, that has a definite chemical composition and an ordered atomic structure. Minerals are the building blocks of rocks and are formed through geological processes.
(ii) Ore: An ore is a type of rock that contains sufficient minerals with important elements, such as metals, that can be extracted profitably. For example, bauxite is an ore of aluminium.
(iii) Gangue: Gangue refers to the unwanted or non-valuable materials, such as sand or rock, that are found with ores during the extraction of metals. They need to be separated during the mining process.
- Name two metals which are found in nature in the free state.
Answer: Gold (Au) and Platinum (Pt) are two metals that are found in nature in their native, free state because they do not easily react with other elements.
- What chemical process is used for obtaining a metal from its oxide?
Answer: The chemical process used for obtaining a metal from its oxide is called reduction. This involves removing the oxygen from the metal oxide, often by heating it with a reducing agent like carbon or through electrolysis. For example, iron oxide (Fe₂O₃) is reduced to iron (Fe) in a blast furnace using carbon (coke).
NCERT Class 10 Science Chapter 3 Intext Question Answer Page Number 55
- Metallic oxides of zinc, magnesium and copper were heated with the following metals.
| Metal | Zinc | Magnesium | Copper |
| Zinc oxide Magnesium oxide Copper oxide |
In which cases will you find displacement reactions taking place?
Answer:
| Metal | Zinc | Magnesium | Copper |
| Zinc oxide Magnesium oxide Copper oxide | No Reaction No Reaction Displacement | Displacement No Reaction Displacement | No Reaction No Reaction No Reaction |
- Which metals do not corrode easily?
Answer: Less reactive metals do not corrode easily. Examples: Gold, Platinum, Silver, Aluminium, Titanium, and Stainless Steel.
- What are alloys?
Answer: Alloys are mixtures of two or more metals, or a metal and another element, designed to enhance certain properties compared to their individual components. Alloys are typically created to improve characteristics such as strength, ductility, corrosion resistance, and conductivity.
NCERT Class 10 Science Chapter 3 Exercise Questions
- Which of the following pairs will give displacement reactions?
(a) NaCl solution and copper metal
(b) MgCl2 solution and aluminium metal
(c) FeSO4 solution and silver metal
(d) AgNO3 solution and copper metal.
Answer: (d) AgNO₃ solution and copper metal
- Which of the following methods is suitable for preventing an iron frying pan from rusting?
(a) Applying grease
(b) Applying paint
(c) Applying a coating of zinc
(d) All of the above.
Answer: (c) Applying a coating of zinc
- An element reacts with oxygen to give a compound with a high melting point. This compound is also soluble in water. The element is likely to be
(a) calcium
(b) carbon
(c) silicon
(d) iron.
Answer: (a) calcium
- Food cans are coated with tin and not with zinc because
(a) zinc is costlier than tin.
(b) zinc has a higher melting point than tin.
(c) zinc is more reactive than tin.
(d) zinc is less reactive than tin.
Answer: (c) zinc is more reactive than tin.
- You are given a hammer, a battery, a bulb, wires and a switch.
(a) How could you use them to distinguish between samples of metals and non-metals?
(b) Assess the usefulness of these tests in distinguishing between metals and non-metals.
Answer: (a) With the hammer, we can beat the sample.If it can be beaten into thin sheets, then it is a metal otherwise a non-metal. Similarly, we can use the battery, bulb, wires, and a switch to set up a circuit with the sample. If the sample conducts electricity, then it is a metal otherwise a non-metal.
(b)The above tests are based on the physical properties of metals and non-metals. No chemical reactions are involved in these tests.
- What are amphoteric oxides? Give two examples of amphoteric oxides.
Answer: Amphoteric oxides are oxides that can react with both acids and bases to form salts and water. This property allows them to act either as an acid or a base, depending on the conditions of the reaction. For example: aluminium oxide (Al2O3), zinc oxide (ZnO)
- Name two metals which will displace hydrogen from dilute acids, and two metals which will not.
Answer: Metals that will displace hydrogen from dilute acids:
- Zinc
- Magnesium
Metals that will not displace hydrogen from dilute acids:
- Copper
- Gold
- In the electrolytic refining of a metal M, what would you take as the anode, the cathode and the electrolyte?
Answer: Anode: The impure metal M is used as the anode. During the electrolysis, metal ions dissolve from the anode into the electrolyte.
Cathode: A pure metal M is used as the cathode. Metal ions from the electrolyte are deposited onto the cathode, forming pure metal.
Electrolyte: The electrolyte is typically a solution of the metal salt, such as MCl2 or MSO4, which contains the metal ions that will be deposited onto the cathode.
- Pratyush took sulphur powder on a spatula and heated it. He collected the gas evolved by inverting a test tube over it, as shown in figure below.
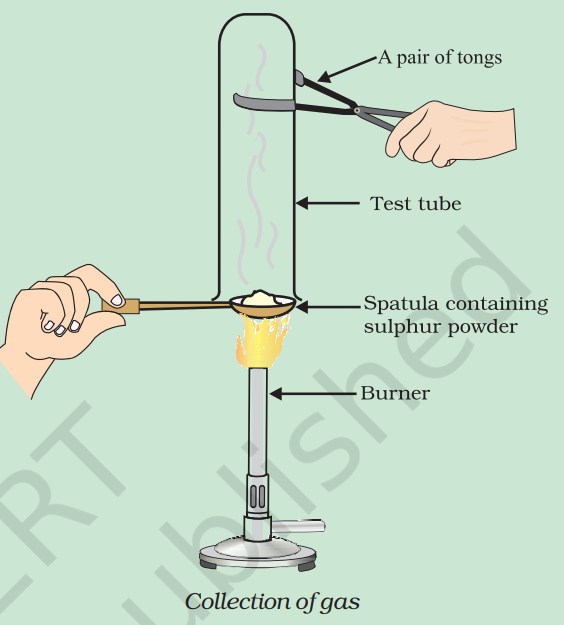
(a) What will be the action of gas on
(i) dry litmus paper?
(ii) moist litmus paper?
(b) Write a balanced chemical equation for the reaction taking place.
Answer: (i) There will be no action on dry litmus paper.
(ii) Since the gas is sulphur dioxide, it turns moist blue litmus paper to red
because sulphur dioxide reacts with moisture to form sulphurous acid which is acidic in nature .
(b)Balanced chemical equation for the reaction taking place on burning of sulphur
S+O2→SO2
Balanced chemica lequation for sulphur dioxide reacting with moisture:
SO2+H2O→H2SO3
- State two ways to prevent the rusting of iron.
Answer: 1. Galvanisation: This involves coating iron with a layer of zinc. The zinc acts as a sacrificial metal, corroding in place of the iron and thus preventing rust formation.
- Applying Protective Coatings: Painting or applying oil or grease to iron surfaces creates a barrier that prevents moisture and oxygen from coming into contact with the metal, significantly reducing the risk of rusting.
- What type of oxides are formed when non-metals combine with oxygen?
Answer: Non-metallic oxides are formed when non-metals combine with oxygen. These oxides are typically acidic oxides.
Here are some examples:
- Carbon dioxide (CO₂)
- Sulfur dioxide (SO₂)
- Nitrogen dioxide (NO₂)
- Phosphorus pentoxide (P₂O₅)
- Give reasons
(a) Platinum, gold and silver are used to make jewellery.
(b) Sodium, potassium and lithium are stored under oil.
(c) Aluminium is a highly reactive metal, yet it is used to make utensils for cooking.
(d) Carbonate and sulphide ores are usually converted into oxides during the process of
Extraction.
Answer: (a) Platinum, Gold, and Silver for Jewelry
Platinum, gold, and silver are highly prized for their lustrous appearance, malleability, ductility, and resistance to corrosion. These properties make them ideal for crafting intricate jewelry designs that retain their beauty and value over time. Additionally, these metals are relatively rare, contributing to their desirability and high market value.
(b) Sodium, Potassium, and Lithium Stored Under Oil
Sodium, potassium, and lithium are highly reactive metals that readily react with air and water. To prevent them from reacting and catching fire, they are stored under oil. The oil creates a protective layer that shields the metals from contact with the atmosphere.
(c) Aluminium Utensils Despite Reactivity
Although aluminium is a highly reactive metal, it forms a thin, protective layer of aluminium oxide (Al₂O₃) on its surface when exposed to air. This oxide layer is impermeable and resistant to further corrosion, making aluminium safe for use in cooking utensils. Additionally, aluminium is a good conductor of heat, which ensures even cooking and efficient heat transfer.
(d) Conversion of Carbonate and Sulphide Ores to Oxides
Carbonate and sulphide ores are often converted to oxide ores during the extraction process because oxides are generally easier to reduce to metals.
- You must have seen tarnished copper vessels being cleaned with lemon or tamarind juice. Explain why these sour substances are effective in cleaning the vessels.
Answer: The sour substances in lemon and tamarind juice, which are primarily citric and tartaric acids, respectively, react with the copper oxide tarnish on the vessel. This reaction forms a soluble copper salt, which can be easily washed away with water. This process effectively removes the tarnish and restores the vessel's shine.
- Differentiate between metal and non-metal on the basis of their chemical properties.
Answer:
| Property | Metals | Non-Metals |
| Reaction with Oxygen | Form basic oxides (e.g., sodium oxide, calcium oxide) | Form acidic oxides (e.g., carbon dioxide, sulfur dioxide) |
| Reaction with Water | Most metals react with water to form metal hydroxides and hydrogen gas (e.g., sodium reacts with water to form sodium hydroxide and hydrogen) | Generally do not react with water (except for a few, like chlorine) |
| Reaction with Acids | Generally do not react with dilute acids (except for a few, like zinc and iron) | React with dilute acids to form salts and hydrogen gas (e.g., zinc reacts with hydrochloric acid to form zinc chloride and hydrogen) |
| Reaction with Bases | Do not react with bases | Do not react with bases (except for a few, like chlorine) |
| Formation of Ions | Tend to lose electrons to form positive ions (cations) | Tend to gain electrons to form negative ions (anions) |
| Conductivity | Good conductors of electricity and heat | Poor conductors of electricity and heat (except for graphite) |
| Malleability and Ductility | Can be hammered into thin sheets and drawn into wires | Brittle and cannot be easily shaped |
| Lustrous | Generally shiny and lustrous | Usually dull and non-lustrous |
- A man went door to door posing as a goldsmith. He promised to bring back the glitter of old and dull gold ornaments. An unsuspecting lady gave a set of gold bangles to him which he dipped in a particular solution. The bangles sparkled like new but their weight was reduced drastically. The lady was upset but after a futile argument the man beat a hasty retreat. Can you play the detective to find out the nature of the solution he had used?
Answer: He must have dipped the gold metal in the solution of aqua regia —3:1 mixture of conc.HCl and conc.HNO3. Aqua regia is a fuming, highly corrosive liquid. It dissolves gold in it. After dipping the gold ornaments in aqua regia, the outer layer ofgold gets dissolved and the inner shiny layer appears.That is why the weight of goldornament reduced.
- Give reasons why copper is used to make hot water tanks and not steel (an alloy of iron).
Answer: Copper does not react with cold water, hot water, or steam. On the other hand, iron reacts with steam. If the hot water tanks are made of steel, then iron (in steel)would react vigorously with the steam formed from hot water. Thus, steel cannot be used for this purpose.
3Fe+4H2O→Fe3O4+4H2
| Download NCERT Class 10 Chapter 3 Metals and Non-Metals Solutions PDF |
Also Check:
Comments
All Comments (0)
Join the conversation