What Are Atomic Orbitals: Atomic orbitals a concepts derived from quantum mechanics to describe the probability of finding an electron around an atom’s nucleus.
Orbitals Chemistry
- There are four kinds of orbitals, denoted by s,p, d and f each with a different shape.
- Out of the four, s and p orbitals are considered because these orbitals are the most common in chemistry.
- A s-orbital is said to be spherical with its nucleus in the centre, the p-orbital is dumbbell-shaped and four of the five d-orbitals are cloverleaf-shaped.
- The fifth d-orbital is like an elongated dumbbell.
- The orbitals in an atom can be organised into different layers and electron shells.
- Well, an atom can have many different numbers of orbitals. They can be categorised based on size, shape and orientation.
- A smaller size orbital means that there is a greater chance of getting an electron near the nucleus.
The Orbital Wave Function
- The Orbtial Wave Function is a mathematical function used for representing the coordinates of an electron. It can be represented by ϕ.
- If we talk about the square of the orbital wave function, then it represents the probability of finding an electron.
- This wave function also helps us to draw the boundary surface diagrams.
- Boundary surface diagrams can help us to understand the shape of orbitals.
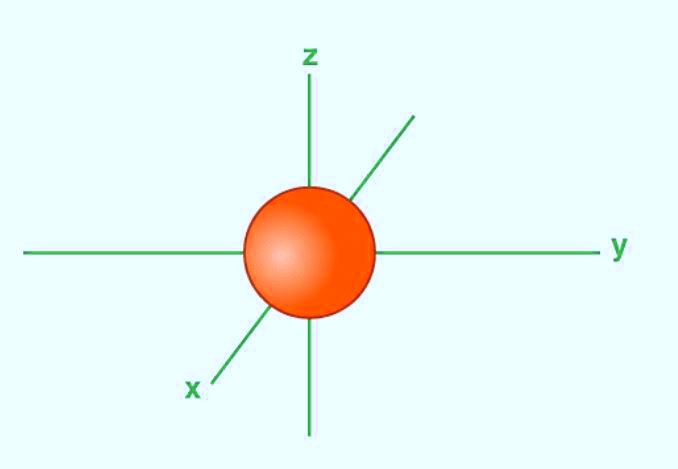
The Shape Of s-orbitals
- The boundary surface diagram for the s-orbital looks like a sphere. It has the nucleus as its centre which we can see in two dimensions in a circle.
- The s-orbitals are spherically symmetric having the probability of finding an electron at a given distance that is equal in all the directions.
- The size of the s-orbital increases with the increase in the value of a principal quantum number.
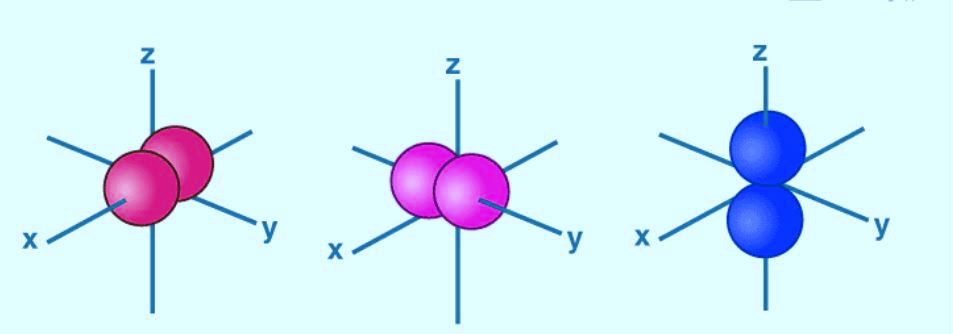
The Shape of p Orbitals
- This orbital consists of two sections better known as lobes, which lie on either side of the plane passing through the nucleus.
- The three p-orbitals are identical in terms in size of shape, size and energy.
- As the lobes lie along the x, y or z-axis, three orbitals are given the designations as 2px, 2py, and 2pz.
- The energy of p-orbitals increases with an increase in the principal quantum number.
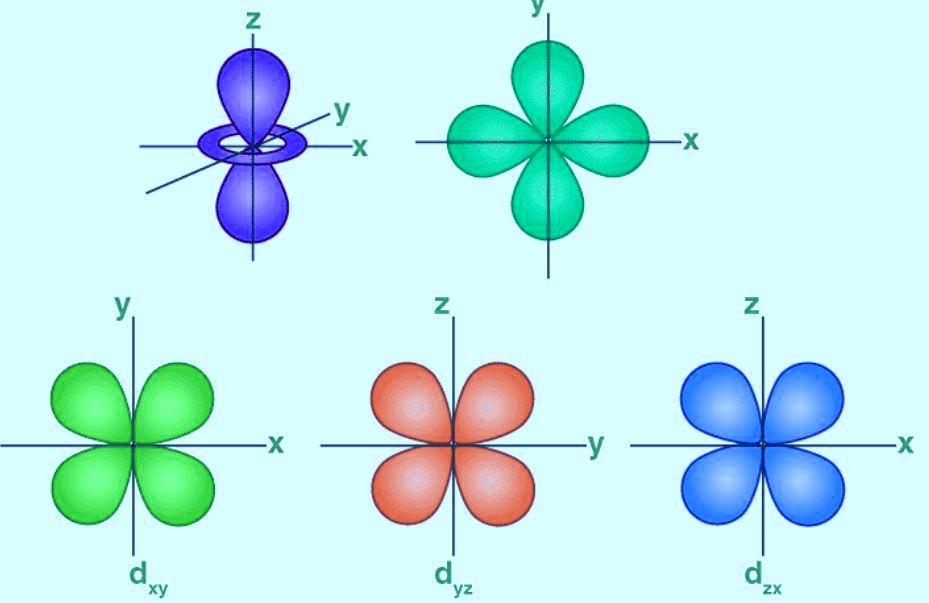
The Shape of d Orbitals
- There are five d-orbitals and the magnetic orbital quantum number for d-orbitals is given as (-2,-1,0, 1,2).
- The orbitals can be designated as dxy, dyz, dxz, dx2–y 2 and dz2.
- The shapes of the first four d-orbitals are similar to each other.
Video Lessons For Students
Comments
All Comments (0)
Join the conversation