In every kitchen, the pressure cooker stands out as both a time-saving hero and, at times, a source of unease. Known for tenderizing the toughest meats and cutting cooking time in half, this humble pot uses the power of steam under pressure to cook food efficiently. But behind that hissing lid lies an age-old scientific principle.
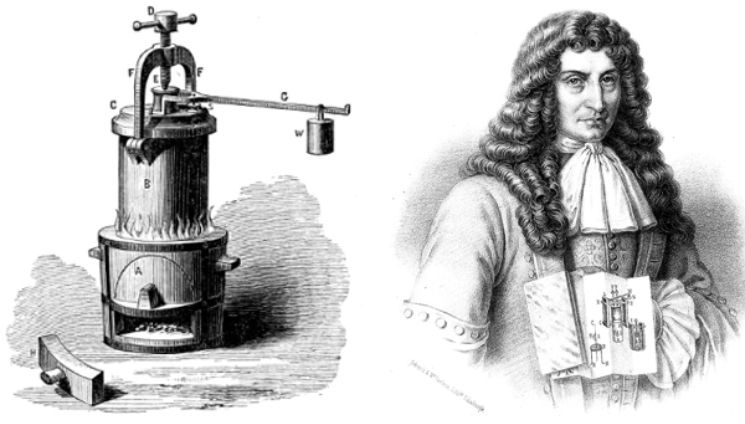
Did you know this common kitchen appliance has roots tracing back to the 17th century? In 1679, French physicist Denis Papin, a pioneer in steam power, invented the steam digester—an early prototype of the modern pressure cooker. Papin’s design used a tightly sealed pot to trap steam, while increasing temperature and pressure, which allowed food to cook faster. His invention laid the foundation not just for pressure cookers, but also for the development of steam engines.
In this article, we will explore how pressure cookers work, what scientific laws or principles are in action, and finally, why pressure cookers explode.
How A Pressure Cooker Works?
To understand why pressure cookers explode, we first need to dive into how a pressure cooker works—and the science that makes it so efficient in your kitchen.
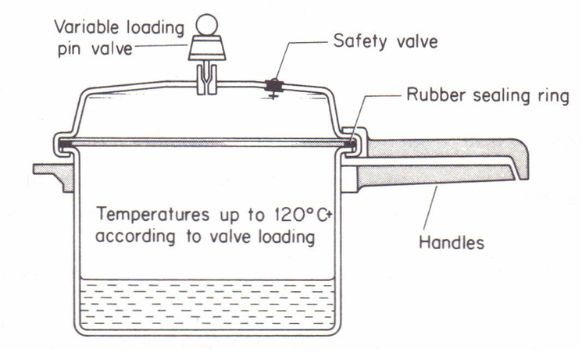
A pressure cooker is essentially a sealed cooking vessel designed to trap steam generated from boiling liquids inside. As the temperature rises, the liquid (usually water or broth) starts to boil and turns into steam. But unlike in an open pot, this steam has nowhere to escape. It begins to build up, creating high internal pressure inside the cooker.
So why exactly does pressure rise with temperature in a sealed vessel—and how does that make your dal cook faster and evenly? The answer lies in gas laws, particularly Gay-Lussac’s Law and the Ideal Gas Law, both of which form the scientific foundation of every pressure cooker. Another principle at play that ensures that your food cooks evenly is convection.
Let’s break down.
Also read: Science Behind It: How Do Thermometers Measure Temperature?
Gay-Lussac’s Law
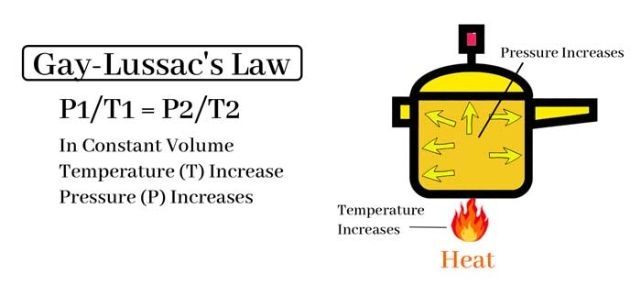
Gay-Lussac’s Law tells us that when the volume and the amount of gas remain constant, the pressure of a gas increases with temperature. In a pressure cooker, the volume is fixed (you are not expanding the pot), and the steam produced from boiling water has nowhere to escape.
As you heat the cooker, the temperature inside rises, and so does the pressure. This pressurized environment leads to an important shift: water now boils at a much higher temperature than its normal 100°C (212°F). In fact, inside a typical pressure cooker, it can boil at around 120°C (250°F)—cooking food faster and more efficiently.
Also read: Science Behind It: How Do Magnets Work?
Ideal Gas Law
Now, let’s bring in the Ideal Gas Law: PV = nRT, where:
-
P = pressure
-
V = volume
-
n = number of gas particles (in this case, steam molecules)
-
R = gas constant
-
T = temperature
In this sealed cooking environment, as temperature (T) increases, and volume (V) remains constant, pressure (P) must increase to maintain the balance. This is the precise equation at work inside your everyday kitchen pressure cooker.
The result? Steam pressure raises the boiling point of water, allowing the food inside to cook at a much higher temperature than in open-air boiling. That’s why even tough cuts of meat or hard lentils soften in minutes—something that would otherwise take hours using conventional boiling methods.
Principle of Convection
Convection is the transfer of heat through the movement of fluids—in this case, steam. As the steam circulates, it carries heat uniformly throughout the cooker. This continuous movement allows the heat to surround the food from all sides, ensuring that even dense or thick items cook consistently without burning or needing to be stirred.
That’s a lot of science going on—and like in any science lab, when pressure and heat are at play, things can go wrong. Just as these principles make cooking faster and more efficient, they also set the stage for potential mishaps. So how does a pressure cooker explode?
Let’s break it down.
Also read: Science Behind It: Why Does It Rain Diamonds On Uranus and Neptune?
Why Do Pressure Cookers Explode?
Despite significant advancements in safety mechanisms—like locking lids, pressure indicators, and steam release valves—pressure cooker explosions can still happen, though they’re rare. These explosions usually occur due to a failure in handling, maintenance, or basic design. Here are the main reasons why:
- Overheating: When a pressure cooker is exposed to continuous high heat, especially without sufficient liquid, it can cause extreme pressure buildup, damaging internal components and increasing the risk of explosion.
- Low Water Levels: Water is essential for creating steam. If the water level is too low, steam generation is compromised, leading to dry heating, overheating, and unsafe pressure spikes.
- Overfilling the Cooker: Filling the cooker beyond the recommended limit leaves less space for steam to expand. This restricts pressure control and can cause clogging or dangerous internal pressure.
- Opening the Cooker Too Soon: Opening the lid before pressure is fully released is one of the most common causes of accidents. It causes a rapid and violent escape of steam and hot contents.
- Faulty Pressure Release Valves: If the release valve is blocked by food residue or malfunctions due to poor maintenance, steam cannot escape properly, resulting in uncontrolled pressure buildup.
- Defective or Worn Gaskets: The rubber sealing ring (gasket) is crucial for maintaining pressure. If it’s damaged, cracked, or too old, it may leak steam or fail to regulate internal pressure safely.
- Old or Poor-Quality Cookers: Older models or inexpensive cookers may lack essential safety mechanisms like pressure indicators, locking lids, and backup valves, making them more prone to malfunction.
Final Whistle!
We hope you found this article both useful and eye-opening. From the 17th-century invention of the steam digester to the gas laws that power your modern pressure cooker, it’s fascinating how everyday appliances are grounded in real science. While pressure cookers are efficient and safe when used properly, understanding how they work—and why they sometimes explode—can help you use them more confidently. Got a topic you’re curious about? Tell us in the comments what you’d like us to explain next in The Science Behind It!
Also Read: Science Behind It: Why The Sun Never Sets In Norway?
Comments
All Comments (0)
Join the conversation