AHSEC Assam Board Class 12 Chemistry Syllabus 2024-25: Assam Board has uploaded the current syllabus for the academic session 2024-25 on its official website. In this article, students can find the syllabus for Class 12 Chemistry syllabus 2024-25. This syllabus can help the students to prepare for the exam well in advance. The subject consists of theory and practical parts. Students will be able to take accurate references throughout the academic year. The syllabus is the key for students to get good marks and prepare well for exams. Read the complete article for better understanding.
Check: AHSEC Assam Board Class 12 Syllabus 2024-25 All Subjects
Assam Board Class 12 Chemistry Syllabus 2024-25: Course Structure
The course structure for the Chemistry syllabus is attached below for easy reference, students are advised to click on the link below to download the complete course structure and syllabus:

Assam Board Class 12 Chemistry Syllabus 2024-25
To get the direct link to download the complete syllabus in PDF format for class 12 Chemistry AHSEC Assam Board syllabus 2024-25, click on the link below mentioned:
Unit 1: Solutions
Types of solutions, expression of concentration of solutions of solids in liquids, solubility of gases in
liquids, solid solutions, Raoult's law, colligative properties - relative lowering of vapour pressure,
elevation of boiling point, depression of freezing point, osmotic pressure, determination of molecular
masses using colligative properties, abnormal molecular mass, Van't Hoff factor.
Unit 2: Electrochemistry
Redox reactions, EMF of a cell, standard electrode potential, Nernst equation and its application to
chemical cells, Relation between Gibbs energy change and EMF of a cell, conductance in electrolytic
solutions, specific and molar conductivity, variations of conductivity with concentration, Kohlrausch's
Law, electrolysis and law of electrolysis (elementary idea), dry cell-electrolytic cells and Galvanic cells,
lead accumulator, fuel cells, corrosion.
Unit 3: Chemical Kinetics
Rate of a reaction (Average and instantaneous), factors affecting rate of reaction: concentration,
temperature, catalyst; order and molecularity of a reaction, rate law and specific rate constant,
integrated rate equations and half-life (only for zero and first order reactions), concept of collision
theory (elementary idea, no mathematical treatment), activation energy, Arrhenius equation.
Unit 4: d and f Block Elements
General introduction, electronic configuration, occurrence and characteristics of transition metals,
general trends in properties of the first row transition metals – metallic character, ionization enthalpy,
oxidation states, ionic radii, colour, catalytic property, magnetic properties, interstitial compounds,
alloy formation, preparation and properties of K2Cr2O7 and KMnO4.
Lanthanoids - Electronic configuration, oxidation states, chemical reactivity and lanthanoid
contraction and its consequences.
Actinoids - Electronic configuration, oxidation states and comparison with lanthanoids.
Check: Periodic Table
Unit 5: Coordination Compounds
Coordination compounds - Introduction, ligands, coordination number, colour, magnetic properties
and shapes, IUPAC nomenclature of mononuclear coordination compounds. Bonding, Werner's
theory, VBT, and CFT; structure and stereoisomerism, importance of coordination compounds (in
qualitative analysis, extraction of metals and biological system).
Unit 6: Haloalkanes and Haloarenes.
Haloalkanes: Nomenclature, nature of C–X bond, physical and chemical properties, optical rotation
mechanism of substitution reactions.
Haloarenes: Nature of C–X bond, substitution reactions (Directive influence of halogen in
monosubstituted compounds only).
Uses and environmental effects of dichloromethane, trichloromethane, tetrachloromethane,
iodoform, freons, DDT.
Unit 7: Alcohols, Phenols and Ethers
Alcohols: Nomenclature, methods of preparation, physical and chemical properties (of primary
alcohols only), identification of primary, secondary and tertiary alcohols, mechanism of dehydration,
uses with special reference to methanol and ethanol.
Phenols: Nomenclature, methods of preparation, physical and chemical properties, acidic nature of
phenol, electrophillic substitution reactions, uses of phenols.
Ethers: Nomenclature, methods of preparation, physical and chemical properties, uses.
Unit 8: Aldehydes, Ketones and Carboxylic Acids
Aldehydes and Ketones: Nomenclature, nature of carbonyl group, methods of preparation, physical
and chemical properties, mechanism of nucleophilic addition, reactivity of alpha hydrogen in
aldehydes, uses.
Carboxylic Acids: Nomenclature, acidic nature, methods of preparation, physical and chemical
properties; uses.
Unit 9: Amines
Amines: Nomenclature, classification, structure, methods of preparation, physical and chemical
properties, uses, identification of primary, secondary and tertiary amines.
Diazonium salts: Preparation, chemical reactions and importance in synthetic organic chemistry.
Check: Amines
Unit 10: Biomolecules
Carbohydrates - Classification (aldoses and ketoses), monosaccahrides (glucose and fructose), D-L
configuration oligosaccharides (sucrose, lactose, maltose), polysaccharides (starch, cellulose,
glycogen); Importance of carbohydrates.
Proteins -Elementary idea of - amino acids, peptide bond, polypeptides, proteins, structure of proteins
- primary, secondary, tertiary structure and quaternary structures (qualitative idea only), denaturation
of proteins; enzymes. Hormones - Elementary idea excluding structure.
Vitamins - Classification and functions.
Nucleic Acids: DNA and RNA.
Assam Board Class 12 Chemistry Syllabus 2024-25: Practicals
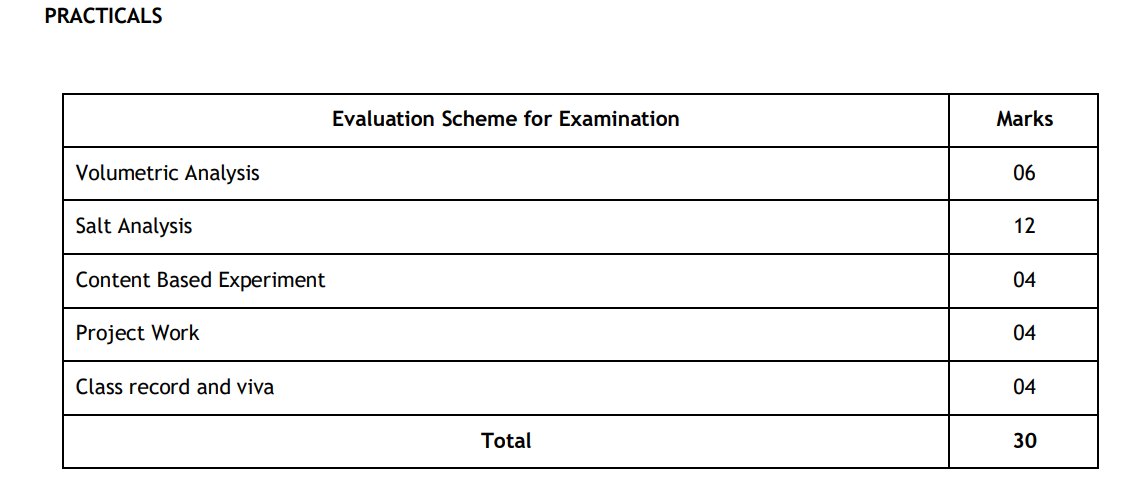
download the complete syllabus for the Assam Board class 12 Chemistry syllabus in PDF format for free, click the link below and get the full syllabus details and bifurcation.
Assam Board Class 12 Chemistry Syllabus 2024-25 Download PDF
Also Check:
Assam Board HS 1st Year Syllabus 2024-25 All Subjects PDF
NCERT Class 12 Revised Textbook PDFs (All Subjects)
Know More:
Comments
All Comments (0)
Join the conversation