Amines are an important class of organic compounds. Students might be aware that adrenaline and ephedrine, which contain secondary amino groups, are used to increase blood pressure. Novocain which is a synthetic amino compound, is used as an anaesthetic in dentistry. Benadryl contains a tertiary amino group.
What are Amines?
Amines are a class of organic compounds which are derived by replacing one or more hydrogen atoms of ammonia molecule by alkyl/aryl group(s). Amines are considered to be the derivatives of ammonia. Amines are often categorised as primary, secondary and tertiary on the basis of the number of hydrogen atoms replaced by alkyl.
When one hydrogen atom of ammonia is replaced by R or Ar, you get a primary amine (RNH2 or ArNH2). When two hydrogen atoms of ammonia or one hydrogen atom of R-NH2 are replaced by another alkyl/aryl(R’) group, you get a secondary amine (R-NHR’). When you replace another hydrogen atom by alkyl/aryl group, it forms a tertiary amine.
Also Check: Electronegativity: Meaning, Periodic Trends in Electronegativities of Elements And More
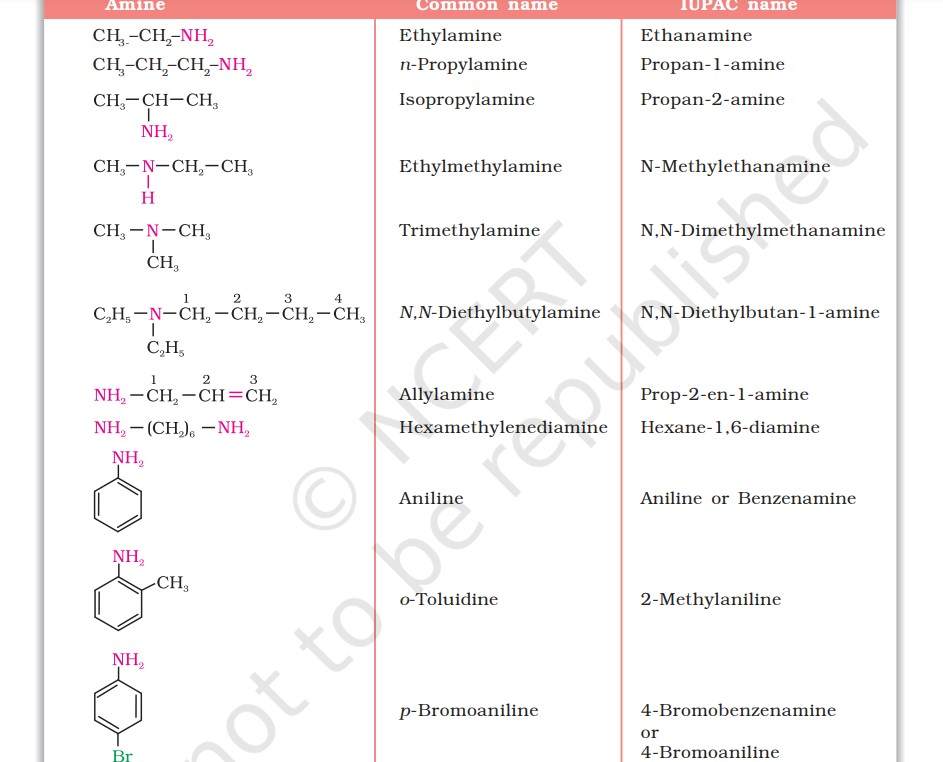
Nomenclature of Amines
In the IUPAC system, amines are known as alkanamines, which is derived by the replacement of ‘e’ of alkane by the word amine. While naming arylamines according to the IUPAC system, suffix ‘e’ of arene is replaced by ‘amine’.
Preparation of Amines
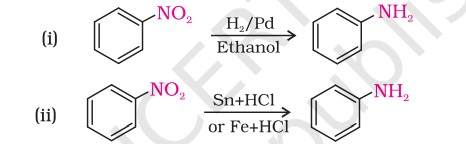
Reduction of nitro compounds- In this method, nitro compounds are reduced to amines by passing hydrogen gas in the presence of divided nickel, palladium or platinum and also by reduction with metals in acidic medium.
Ammonolysis of alkyl halides- In this method, an alkyl on reaction with an ethanolic solution of ammonia undergoes nucleophilic substitution reaction in which the halogen atom is replaced by an amino (–NH2 ) group. This process of cleavage of the C–X bond by ammonia molecule is known as ammonolysis.The primary amine thus obtained behaves as a nucleophile and can further react with alkyl halide to form secondary and tertiary amines, and finally quaternary ammonium salt.
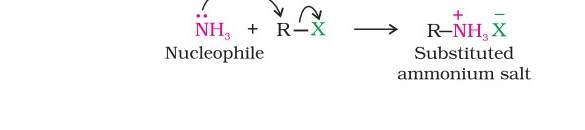
Reduction of nitriles- In this technique, nitriles on reduction with lithium aluminium hydride or catalytic hydrogenation produce primary amines. This reaction is used for the preparation of amines containing one carbon atom more than the starting amine.
![]()
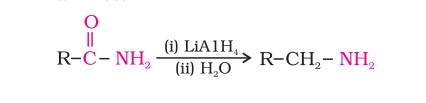
Reduction of amides- Here the amides on reduction with lithium aluminium hydride give amines.
Also Check: Heat Capacity: Specific Heat Capacity of Water, Molar Specific Heat Capacity And More
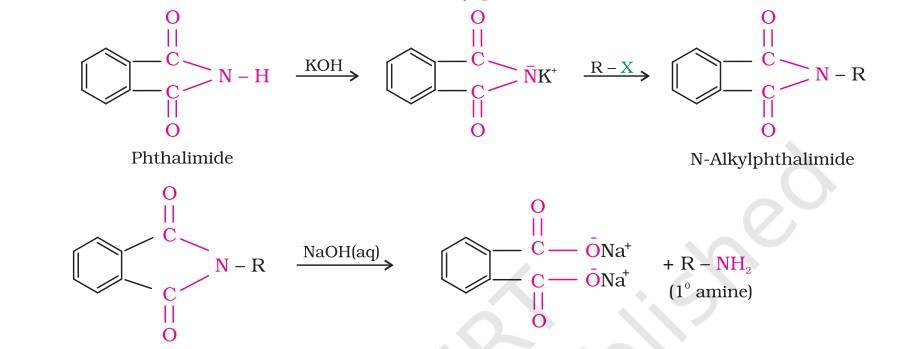
Gabriel phthalimide synthesis-In this technique, Gabriel synthesis is used for the preparation of primary amines. Phthalimide on treatment with ethanolic potassium hydroxide forms potassium salt of phthalimide which on heating with alkyl halide followed by alkaline hydrolysis produces the corresponding primary amine.
Physical Properties of Amines
The lower aliphatic amines are gases with fishy odour and they are soluble in water. The primary amines with three or more carbon atoms are liquid. Higher amines are insoluble in water. Primary and secondary amines are involved in intermolecular association because of hydrogen bonding between nitrogen of one and hydrogen of another molecule. This association does not happen in tertiary amines due to the absence of hydrogen atom available for hydrogen bond formation.
Chemical Properties of Amines
Since amines are basic in nature they react with acids to form salts. There are various chemical reactions through which Amines undergo through processes like alkylation, acylation, carbylamine reactions and others.
Uses of Amines
Amines are widely used in the preparation of dyes.
Animes are used in various painkillers such as demerol and morphine.
Animes act as stimulants for neurotransmitters like serotonin present in our body.
Amines are known as sources of amino acids.
Animes are also used in popular syrups like Benadryl.
CBSE Video Courses for Science for Class 12 Students
Class 12 Science students can study effectively for the exams with the help of video courses prepared by the subject matter experts. These video courses will explain the concepts in a simple and interactive manner which will help learners to understand clearly.
Note: All images have been taken from NCERT Class 12 Chemistry textbook.
Also, check
Ethanol: Meaning, Chemical Formula And Uses
Carboxylic Acids: Definition, Structure, Nomenclature, Physical Properties
Solutions: Meaning, Components, Features, Solute vs Solvent
Domains of the Earth: Biosphere, Lithosphere, Atmosphere, Hydrosphere

Comments
All Comments (0)
Join the conversation