CBSE 2024-25 Competency-Based Questions With Answers: The Central Board of Secondary Education (CBSE) has modified the question paper format for almost all the subjects of Classes 9 to 10. Now 50% of the question paper will be covered by competency-based questions (CBQs).
Here, the CBSE Class 10 Science Chapter 2 Acids, Bases, and Salts competency-based questions are given from all the major topics. These questions are from CBSE experts and thus are best to make you ready for the 2025 Class 10 Board exams. Keep checking and download the PDF.
CBSE Class 10 Science Acids, Bases and Salts Competency-Based Questions
All the questions provided below are from the CBSE document published under the handbook and manual section for the 2025 Class 10 board examination.
| Q. No | Question | Marks | |||||||||||||||
| Multiple Choice Question | |||||||||||||||||
| Q.1 | Hydrangea plants develop blue or pink flowers depending on the availability of aluminium from the soil. When the soil is acidic, aluminium is more available to the roots, resulting in blue flowers. When the soil is alkaline, the availability of aluminium decreases, resulting in pink flowers. The graph below is of the pH of the soil at different sections of a field. 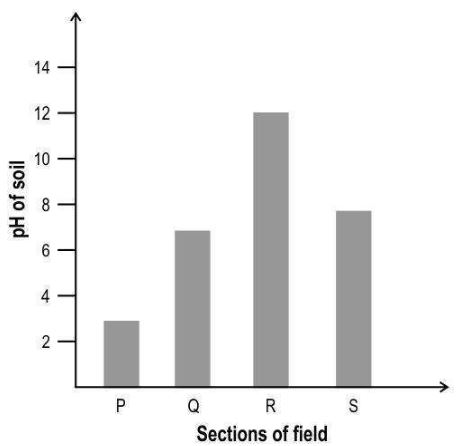
In which section of the field will the flowers on ALL the hydrangea plants definitely be blue in colour and in which section will the flowers on ALL the hydrangea plants definitely be pink in colour? A. W B. X C. Y D. Z | 1 | |||||||||||||||
| Free Response Question/ Subjective Question | |||||||||||||||||
| Q.2 | An excess of carbon dioxide gas is bubbled through lime water. (a) Will the pH of lime water change? If yes, how? Explain your answer. (b) Write the balanced equation for the reaction. | 3 | |||||||||||||||
| Q.3 | Tanu takes 500 mL milk each in two bowls P and Q. She adds curd to both the bowls and baking soda only to bowl Q as shown below. (a) Bowl P - 500 mL milk + 1 teaspoon curd (b) Bowl Q - 500 mL milk + 1 teaspoon curd + 1 teaspoon baking soda In which bowl will the milk form into curd faster? Explain your answer. | 3 | |||||||||||||||
| Q.4 | A solution P is taken in a flask and two drops of phenolphthalein indicator is added to it. The graph below shows how the pH of the mixture changes as a solution Q is added dropwise to the flask with stirring.  (a) Identify the nature of solutions P and Q. (b) What will the colour of the solution in the flask be at points X and Y? (c) Identify the type of reaction taking place in the flask. | 3 | |||||||||||||||
| Q.5 | Aditi adds 1 mole of dilute hydrochloric acid to an aqueous solution of 1 mole of sodium carbonate. (a) Write the balanced equation for the reaction that takes place. (b) How will the colour of a red litmus and a blue litmus paper change when dipped in this mixture? Explain why. | 5 | |||||||||||||||
| Q.6 | The presence of acidic gases in the air increases the rate of corrosion. Furthermore, an increase in temperature can also increase the rate of corrosion. The graph below is created under 4 different conditions (shown below in the table) of temperature and acidic nature of air.
Which of the graphs represents condition 2? (a) P (b) Q (c) R (d) S | 1 | |||||||||||||||
Answer Key & Marking Scheme
| Q. No | Answers | Marks |
| Q.1 | D. Z | 1 |
| Q.2 | (a)
OR -The products formed, namely, calcium carbonate and Calcium hydrogen carbonate are basic salts but less basic than Calcium hydroxide so pH decreases. (b) 0.5 marks each for writing the formula of lime water and the product calcium bicarbonate; 0.5 marks for balancing the equation: Ca(OH)2 + 2 CO2 ---> Ca(HCO3)2 OR Ca(OH)2 + CO2 ---> CaCO3 + H2O CaCO3 + CO2 + H2O ---> Ca(HCO3)2 | 3 |
| Q.3 | The milk will form into curd faster in bowl P.
| 3 |
| Q.4 | (a) 0.5 marks each for the following:
(b) 0.5 marks for each of the following:
(c) neutralisation | 3 |
| Q.5 | (a) 0.5 marks each for writing the formula of each reactant and product: HCl + Na2CO3 ---> NaCl + NaHCO3 (b)
| 5 |
| Q.6 | Graph P | 1 |
Click on the link below to download the free PDF.
| Download CBSE Class 10 Acids, Bases and Salts Competency-Based Questions with Solution 2025 PDF |
Check out the links below for more information.
Also Read:

Comments
All Comments (0)
Join the conversation