CBSE Class 10 Science Sample Paper 2024-25: The Central Board of Secondary Education (CBSE) has released the 2024-25 Class 10 Science sample paper. The sample paper is provided with a solution PDF that can easily be downloaded from the CBSE academic website. This practice question paper follows the latest CBSE Class 10 Science exam pattern and marking scheme. The sample paper for Class 10 Science 2025 comprises questions from all the units as per their weightage mentioned in the syllabus. Scroll down and download the sample paper and marking scheme PDF. Before that, let us first have a look at the question paper design.
Check | CBSE Class 10 Syllabus 2024-25
Recently Released | CBSE Class 10 Date Sheet 2025
CBSE Class 10 Science Question Paper Design 2024-25
Here’s the structure for the question paper based on the information provided in the latest CBSE Class 10 Science sample paper.
| Section | Question Type | Number of Questions | Marks per Question | Total Marks |
| A | MCQs (Simple/Complex) | 16 | 1 | 16 |
| Assertion-Reasoning | 4 | 1 | 4 | |
| B | Short Answer (SA) | 6 | 2 | 12 |
| C | Short Answer (SA) | 7 | 3 | 21 |
| D | Long Answer (LA) | 3 | 5 | 15 |
| E | Source/Case/Passage-based Questions | 3 | 4 | 12 |
| Internal Choice | ~33% across sections | |||
| Total Marks | 80 |
- Competency-based Questions: All sections will allocate 50% of the marks to competency-based questions.
- Internal Choice: Provided for approximately 33% of the questions throughout the paper.
This structure provides a clear breakdown for each section, question type, and their respective marks.
CBSE Class 10 Science Sample Paper 2024-25
The Class 10 Science sample paper for the 2025 board examination is given below. Each question is designed to test students' conceptual and analytical understanding. Solve this paper and check your answers from the marking scheme provided below:
| Section-A Question 1 to 16 are multiple choice questions. Only one of the choices is correct. Select and write the correct choice as well as the answer to these questions. | ||
| 1 | Identify ‘p’, ‘q’ and ‘r’ in the following balanced reaction Heat p Pb (NO3)2(s) → q PbO(s) + r NO2(g) + O2(g) A. 2,2,4 B. 2,4,2 C. 2,4,4 D. 4,2,2 | |
| 2 | Match column I with column II and select the correct option using the given codes. Column I a. A metal that forms amphoteric oxides b. A metal which melts when kept on our palm c. A metal that reacts with nitric acid d. A metal which cannot displace hydrogen from Column II (i) Ga (ii) Au (iii) Al (iv) Mn acids A. a – (ii), b – (i), c – (iii), d – (iv) B. a – (iii), b – (i), c – (iv), d – (ii) C. a – (iv), b – (ii), c – (iii), d – (i) D. a – (iii), b – (ii), c – (i), d – (iv) | |
| 3. | The solution in the given figure is likely to be
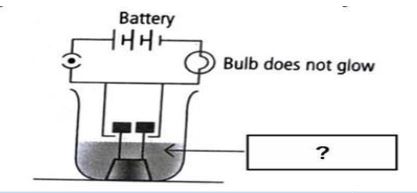
- - - - - - - - - - - - - - For Visual Impaired Students Which among the following is considered as the strongest electrolyte?
| |
| 4 | An aqueous solution ‘A’ turns the phenolphthalein solution pink. On addition of an aqueous solution ‘B’ to ‘A’, the pink colour disappears. Which of the following statement is true for the solutions ‘A’ and ‘B’.
|
| 5 | When 50g of lead powder is added to 300 ml of blue copper sulphate solution, after a few hours, the solution becomes colourless. This is an example of
|
| 6 | The electronic configuration of three elements X, Y and Z are X- 2, 8, 7; Y- 2, 8, 2; and Z - 2, 8
|
| 7 | Which of the following is an endothermic reaction?
|
| 8 | During cellular oxidation of Glucose, ATP is produced along with formation of other products in this reaction. Which of the following events is associated with production of maximum ATP molecules per molecule of Glucose during this process? Synthesis of
| 1 |
| 9 | During which of the following stages of the circulation of blood in a normal human being, the oxygenated blood is pumped to all parts of the body?
| 1 |
| 10 | Which of the following adaptations in herbivores helps in digestions of cellulose?
| 1 |
| 11 | There was a cerebellar dysfunction in a patient. Which of the following activities will get disturbed in this patient as a result of this?
| 1 |
| 12 | In snails individuals can begin life as male and depending on environmental conditions they can become female as they grow. This is because
| 1 |
| 13 | In the following cases, a ray is incident on a concave mirror. In which case is the angle of incidence equal to zero?
| 1 |
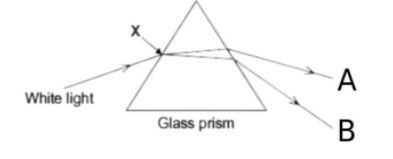
| 14 | Choose the correct option for the colour of rays for A and B. |
↓
↓
To download the complete question paper and its solutions, click on the links below:
Check out the other important links from the section below:
Also Check:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CBSE Class 10 Sample Papers for Board Exam 2024 with Solutions |
Comments
All Comments (0)
Join the conversation