Assam Board SEBA Class 10 Science Sample Paper 2024-25, Download In PDF: The Secondary Education Board Of Assam has released the latest sample papers for High School Leaving Certificate Students (HSLC). The sample papers are available for main subjects such as Science, Mathematics, English and Social Studies.
Students can visit the official website of the Assam board and can download the PDF from the website. At the end of the article, we are also providing the link for students to download the sample papers PDF for free.
| Q.NOS | QUESTIONS | MARKS(approx.) |
| CHAPTER-1 | ||
| SECTION - A | ||
| 1 | Identify the product which represents the solid state in the above reaction. | 1 |
| 2 | The colour of the solution observed after 30 minutes of placing zinc metal in copper sulphate solution is a) Blue b) Colourless c) Dirty green d) Reddish Brown | 1 |
| 3 | In the redox reaction MnO2 + 4HCl → MnCl2 + 2H2O + Cl2 (a) MnO2 is reduced to MnCl2 & HCl is oxidized to H2O (b) MnO2 is reduced to MnCl2 & HCl is oxidized to Cl2 (c) MnO2 is oxidized to MnCl2 & HCl is reduced to Cl2 (d) MnO2 is oxidized to MnCl2 & HCl is reduced to H2O | 1 |
| 4 | Which of the following is the correct observation of the reaction shown in the above setup? (c) Magnesium ribbon burns with brilliant white light.
(d) Reddish brown gas with a smell of burning Sulphur has evolved. | 1 |
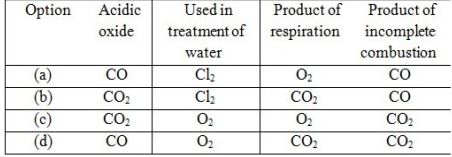
| 5 | With the reference to four gases CO2, CO, Cl2 and O2, which one of the options in the table is correct? | 1 |
| 6 | On placing a copper coin in a test tube containing green ferrous sulphate solution, it will be observed that the ferrous sulphate solution (a) turns blue, and a grey substance is deposited on the copper coin. (b) turns colourless and a grey substance is deposited on the copper coin. (c) turns colourless and a reddish-brown substance is deposited on the copper coin. (d) remains green with no change in the copper coin. | 1 |
| 7 | Reema took 5ml of Lead Nitrate solution in a beaker and added approximately 4ml of Potassium Iodide solution to it. What would she observe? a) The solution turned red. b) A yellow precipitate was formed. c) A white precipitate was formed. d) The reaction mixture became hot. | 1 |
| 8 | Which of the following correctly represents a balanced chemical equation? b) 3Fe(s) + 4H2O(g) → Fe3O4 (s) + 4H2(g) c) 3Fe(s) + H2O(g) → Fe3O4 (s) + H2(g) d) 3Fe(s) + 4H2O(g) → Fe3O4 (s) + H2(g) | 1 |
| 9 | The chemical reaction between copper and oxygen can be categorized as: a) Displacement reaction b) Decomposition reaction c) Combination reaction d) Double displacement reaction | 1 |
| 10 | Why is it important to balance a skeletal chemical equation? a) To verify the law of conservation of energy. b) To verify the law of constant proportion. c) To verify the law of conservation of mass. d) To verify the law of conservation of momentum. | 1 |
| SECTION - B | ||
| 11 | A clear solution of slaked lime is made by dissolving Ca(OH)2in an excess of water. This solution is left exposed to air. The solution slowly goes milky as a faint white precipitate form. Explain why a faint white precipitate forms, and support your response with the help of a chemical equation. | 2 |
| 12 | Identify the correct option from the given table which represents the type of reactions occurring in step 1 and step 2. | 2 |
| SECTION - C | ||
| 13 | i)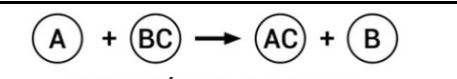 ii) 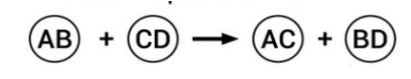 Identify the types of reaction mentioned above in (i) and (ii). Give one example for each type in the form of a balanced chemical equation. | 3 |
| 14 | A reddish-brown metal ‘X’, when heated in air, gives a black compound ‘Y’, which when heated in the presence of H₂ gas gives ‘X’ back. ‘X’ is refined by the process of electrolysis; this refined form of ‘X’ is used in electrical wiring. Identify ‘X’ and ‘Y’. Draw a well-labelled diagram to represent the process of refining ‘X’. | 3 |
How To Download HSLC Sample Papers?
If you are also an Assam board student, then you can see these steps to download the sample papers.
Step 1: Visit the official website of the Secondary Education Board of Assam (SEBA).
Step 2: Click on the download option that will be available on the top bar.
Step 3: Check the link for the PDFs that are available on the website.
Step 4: Check for the sample papers with recent dates and then, click on the download option.
Step-5: Then, save the PDF for further use.
To download the full PDF for all sample papers, check the link below.
| Assam Board HSLC Class 10 Sample Paper Free PDF Download |
Other Related Links

Comments
All Comments (0)
Join the conversation