MCQs for CBSE Class 12 Haloalkanes and Haloarenes: If you are searching for reliable and important study material to help you in your board exam preparations, then Jagran Josh is the best option for you from where you will get the study material created and reviewed by the subject matter experts. Multiple Choice Questions (MCQs) are one part of the study material provided by Jagran Josh. Since the weightage of MCQs has been increased in CBSE Board Exams, it has become essential to prepare these objective questions to score well in the exam.
In this article, we have provided important MCQs for CBSE Class 12 Chemistry Chapter - Haloalkanes and Haloarenes. We have provided all the questions and answers in PDF format as well. These questions are entirely based on the revised CBSE syllabus and are created by experienced faculty. All the questions are provided with answers. Therefore, these questions can be considered one of the most useful resources for board exam preparations. Solve all questions to strengthen your concepts and improve weak areas.
Also Check: CBSE Class 12 Chemistry Syllabus 2023-24
MCQs for CBSE Class 12 Haloalkanes and Haloarenes (Chemistry) 2023-24
1.Which of the following is most reactive towards nucleophilic substitution reaction?
(a) C6H5Cl
(b) CH2=CHCl
(c) ClCH2CH=CH2
(d) CH3CH=CHCl
Answer: (c) ClCH2CH=CH2
2.The most reactive nucleophile among the following is
(a) CH3O–
(b) C6H5O–
(c) (CH3)2CHO–
(d) (CH3)3CO–
Answer:(a) CH3O–
3.The main difference between C – X bond of a haloalkane and a haloarene is
(a) C – X bond in haloalkanes is shorter than haloarenes
(b) In haloalkanes the C attached to halogen in C – X bond is sp3 hybridised while in haloarenes it is sp2 hybridised.
(c) C – X bond in haloalkanes acquires a double bond character due to higher electronegativity of X than haloarenes.
(d) haloalkanes are less reactive than haloarenes due to difficulty in C – X cleavage in haloalkanes.
Answer: (b) In haloalkanes the C attached to halogen in C – X bond is sp3 hybridised while in haloarenes it is sp2 hybridised.
4.Which of the following is a primary halide?
(a) Isopropyl iodide
(b) Secondary butyl iodide
(c) Tertiary butyl iodide
(d) Neohexyl chloride
Answer: (d) Neohexyl chloride
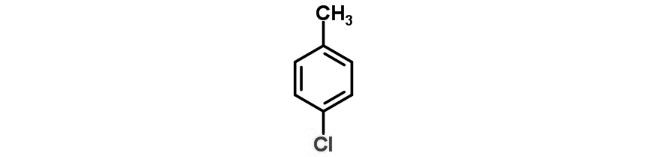
5.Which is the correct IUPAC name for
(a) Methylchlorobenzene
(b) Toluene
(c) 1-Chloro-4-methylbenzene
(d) 1-Methyl-4-chlorobenzene
Answer: (c) 1-Chloro-4-methylbenzene
6.Aryl halides are less reactive towards nucleophilic substitution reactions as compared to alkyl halides due to
(a) formation of a less stable carbonium ion in aryl halides
(b) resonance stabilization in aryl halides
(c) presence of double bonds in alkyl halides
(d) inductive effect in aryl halides
Answer:(b) resonance stabilization in aryl halides
7.p-dichlorobenzene has higher melting point than its o- and m- isomers. Why?
(a) m- dichlorobenzene is more polar than o-isomer
(b) p-isomer has a symmetrical crystalline structure
(c) boiling point of o- isomer is more than p-isomers
(d) All of these are correct
Answer: (b) p-isomer has a symmetrical crystalline structure
8.Chlorobenzene on reaction with NaOH at 300K followed by acidic hydrolysis produces
(a) Phenol
(b) Sodium phenoxide
(c) Benzaldehyde
(d) Benzoic acid
Answer: (a) Phenol
9.Which of the following is most reactive towards aqueous NaOH?
(a) C6H5Cl
(b) C6H5CH2Cl
(c) C6H5Br
(d) BrC6H4Br
Answer:(b) C6H5CH2Cl
10.Which of the following haloalkanes is optically active?
(a) 1-Chloropropane
(b) 1-Bromopropane
(c) 1-Iodopropane
(d) 1-Fluoropropane
Answer: (b) 1-Bromopropane
Download CBSE Class 12 Chemistry MCQs for Haloalkanes and Haloarenes in PDF |
Comments
All Comments (0)
Join the conversation