Term 2 CBSE Class 11 Chemistry Syllabus 2022 PDF is available for download in PDF format. The link to download PDF of CBSE Class 11 Chemistry Syllabus 2022. It contains complete details about Term 2 CBSE Class 11 Chemistry Syllabus 2022 and is important for preparation of the upcoming Term 2 CBSE Class 11 Chemistry exam 2022.
NCERT Books for Class 11 (PDF): All Subjects
Term 2 CBSE Class 11 Chemistry Syllabus 2022: CBSE Class 11 Exam 2022
| S. No | Unit | Marks |
| 1 | States of Matter: Gases and Liquids | 15 |
| 2 | Chemical Thermodynamics | |
| 3 | Equilibrium | |
| 4 | s - Block Elements | 11 |
| 5 | Some p -Block Elements | |
| 6 | Hydrocarbons | 9 |
| Total | 35 |
States of Matter: Gases and Liquids: Three states of matter, intermolecular interactions, types of bonding, melting and boiling points, role of gas laws in elucidating the concept of the molecule, Boyle's law, Charles law, Gay Lussac's law, Avogadro's law, ideal behaviour, empirical derivation of gas equation, Avogadro's number, ideal gas equation and deviation from ideal behaviour.
Chemical Thermodynamics: Concepts of System and types of systems, surroundings, work, heat, energy, extensive and intensive properties, state functions.
First law of thermodynamics -internal energy and enthalpy, measurement of U and H, Hess's law of constant heat summation, enthalpy of bond dissociation, combustion, formation, atomization, sublimation, phase transition, ionization, solution and dilution. Second law of Thermodynamics (brief introduction)
Introduction of entropy as a state function, Gibb's energy change for spontaneous and nonspontaneous processes.
Third law of thermodynamics (brief introduction).
Equilibrium: Equilibrium in physical and chemical processes, dynamic nature of equilibrium, law of mass action, equilibrium constant, factors affecting equilibrium - Le Chatelier's principle, ionic equilibrium- ionization of acids and bases, strong and weak electrolytes, degree of ionization, ionization of poly basic acids, acid strength, concept of pH, buffer solution, solubility product, common ion effect (with illustrative examples).
s -Block Elements: Group 1 and Group 2 Elements -General introduction, electronic configuration, occurrence, anomalous properties of the first element of each group, diagonal relationship, trends in the variation of properties (such as ionization enthalpy, atomic and ionic radii), trends in chemical reactivity with oxygen, water, hydrogen and halogens, uses.
Some p -Block Elements: General Introduction to p -Block Elements
Group 13 Elements: General introduction, electronic configuration, occurrence, variation of properties, oxidation states, trends in chemical reactivity, anomalous properties of first element of the group, Boron - physical and chemical properties.
Group 14 Elements: General introduction, electronic configuration, occurrence, variation of properties, oxidation states, trends in chemical reactivity, anomalous behaviour of first elements.
Carbon-catenation, allotropic forms, physical and chemical properties.
Hydrocarbons: Classification of Hydrocarbons Aliphatic Hydrocarbons:
Alkanes - Nomenclature, isomerism, conformation (ethane only), physical properties, chemical reactions.
Alkenes - Nomenclature, structure of double bond (ethene), geometrical isomerism, physical properties, methods of preparation, chemical reactions: addition of hydrogen, halogen, water, hydrogen halides (Markovnikov's addition and peroxide effect), ozonolysis, oxidation, mechanism of electrophilic addition.
Alkynes - Nomenclature, structure of triple bond (ethyne), physical properties, methods of preparation, chemical reactions: acidic character of alkynes, addition reaction of - hydrogen, halogens, hydrogen halides and water.
Aromatic Hydrocarbons: Introduction, IUPAC nomenclature, benzene: resonance, aromaticity,
chemical properties: mechanism of electrophilic substitution. Nitration, sulphonation, halogenation, Friedel Craft's alkylation and acylation, directive influence of functional group in monosubstituted benzene. Carcinogenicity and toxicity.
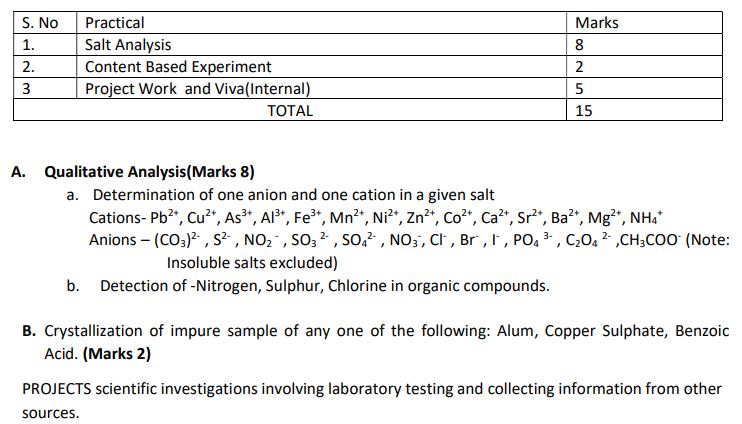
Practical Exams:
Term II: At the end of Term II, a 15-mark Practical would be conducted under the supervision of the subject teacher. This would contribute to the overall practical marks for the subject.
OR
In case the situation of lockdown continues beyond December 2021, a Practical Based Assessment (pen-paper) of 10 marks and Viva 5 marks would be conducted at the end of Term II by the subject teacher. This would contribute to the overall practical marks for the subject.
Comments
All Comments (0)
Join the conversation