Thermodynamics in Physics: Thermodynamics is the branch of physics that deals with the study of heat, work, and energy. It explains how energy is transferred within physical systems and how it affects matter. In this article, we have discussed all key concepts related to thermodynamics in a simple language which will be easier to understand and help clear all easy and complex concepts.
Thermodynamics Definition
Thermodynamics is the science of energy transfer and its effects on the properties of matter. It primarily deals with concepts such as temperature, heat, work, internal energy, and the laws governing these quantities.
Key Concepts of Thermodynamics
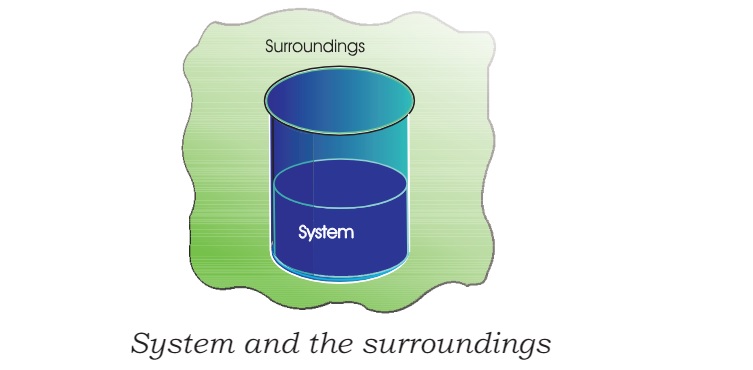
System: In Thermodynamics, the part of the universe under study is termed as System.
Surroundings: Everything outside the system is termed as Surroundings.
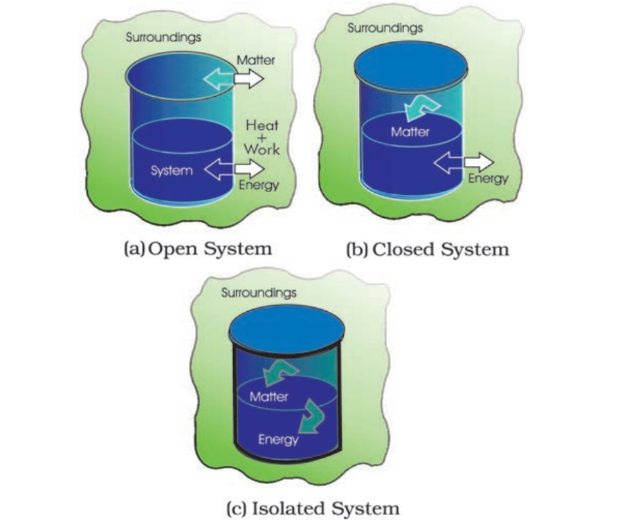
Types of Systems:
- Isolated System: No exchange of energy or matter with surroundings.
- Closed System: Only energy is exchanged with surroundings, not matter.
- Open System: Both energy and matter are exchanged with surroundings.
State Functions:
Properties that depend only on the state of the system like temperature, pressure, volume, internal energy, enthalpy are called state functions.
Laws of Thermodynamics
1. Zeroth Law of Thermodynamics (Law of Thermal Equilibrium)
This law states that if we bring two objects that are initially at different temperatures into physical contact, they eventually achieve thermal equilibrium. During the process of reaching thermal equilibrium, heat is transferred between the objects.
The amount of heat transferred ΔQ is proportional to the temperature difference ΔT between the objects and the heat capacity c of the object.
ΔQ=cΔT
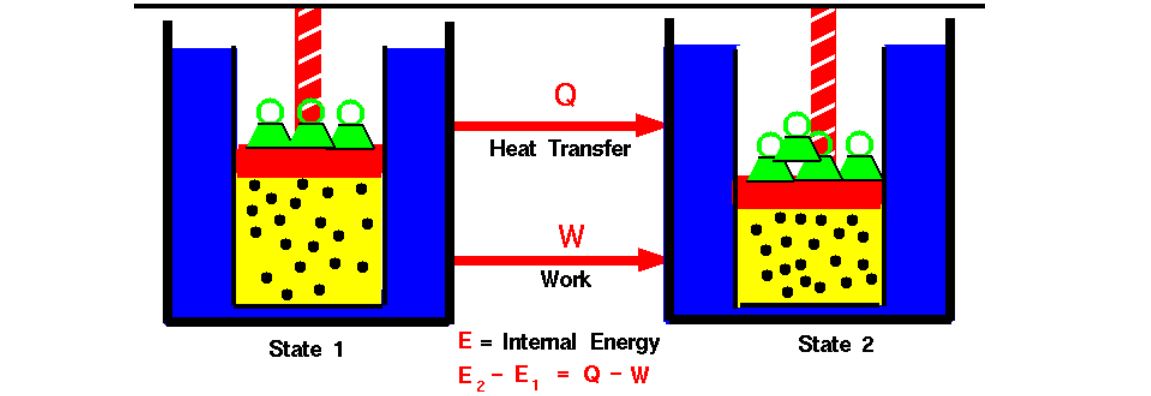
2. First Law of Thermodynamics (Law of Energy Conservation)
Law of Conservation of Energy states that energy can neither be created nor be destroyed. Energy can only be transferred or changed that too from one form to another.
Mathematically: ΔU=Q+W
ΔU = Change in internal energy of the system
Q = Heat added to the system
W = Work done by the system
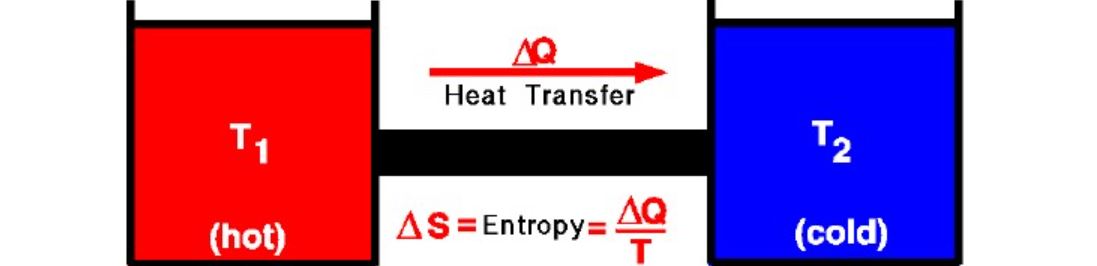
3. Second Law of Thermodynamics
The second law of thermodynamics states that the entropy in an isolated system always increases. The entropy of the universe only increases and never decreases.
Alternatively, the second law of thermodynamics states that heat energy cannot transfer from a body at a lower temperature to a body at a higher temperature without the addition of energy.
Mathematically second law of thermodynamics can be expressed as:
ΔS=ΔQ/T
- ΔS = Change in entropy
- ΔQ= Heat exchanged in a reversible process
- T = Absolute temperature
4. Third Law of Thermodynamics:
As the temperature of a system approaches absolute zero, the entropy of a perfect crystal approaches zero.
Important Equations and Formulas
1.Work Done in Isothermal Process:
For an ideal gas: W=nRTln(Vf/Vi)
n = Number of moles
R = Universal gas constant
T = Temperature
Vf and Vi = Final and initial volumes
2.Heat Transfer (Specific Heat Capacity):
Q=mcΔTQ
m = Mass of the substance
c = Specific heat capacity
ΔT = Change in temperature
3.Enthalpy (H):
H=U+PV
U = Internal energy
P = Pressure
V = Volume
4.Gibbs Free Energy (G):
G = H−TS
H = Enthalpy
T = Temperature
S = Entropy
5.Helmholtz Free Energy (A):
A=U−TS
U = Internal energy
T = Temperature
S = Entropy
Also Read:
Real Life Applications of Thermodynamics
1. Heat Engines and Power Plants
- Internal Combustion Enginesused in cars and other vehicles, converting chemical energy of fuel into mechanical work.
- Thermal Power Plantsconvert thermal energy from burning coal, natural gas, or nuclear reactions into electrical energy.
2. Refrigeration and Air Conditioning
- Refrigerators and freezersuse the principles of thermodynamics to transfer heat from the inside of the unit to the external environment.
- Air Conditionersregulate the temperature and humidity of indoor spaces by transferring heat out of the building.
3. Material Science
- Metallurgy:Processes like smelting and refining metals depend on the principles of thermodynamics to control temperature and energy input.
- Polymer Productioninvolves exothermic and endothermic reactions that are regulated using thermodynamic principles.
4. Biological Systems
- Human Metabolism:The body's conversion of food into energy follows thermodynamic laws, including energy conservation and entropy.
- Enzyme Activity:Thermodynamics helps understand how temperature and energy affect enzyme function and biochemical reactions.
5. Energy Systems
- Solar Panels:Convert sunlight into electrical energy, efficiency is analyzed using thermodynamics.
- Wind Turbines:Efficiency and energy conversion processes are governed by thermodynamic principles.
- Energy Storage:Batteries store and release energy based on thermodynamic principles, crucial for developing efficient energy storage systems.
6. Daily Life
- Cooking:Thermodynamics explains how heat transfers from the stove to the food, affecting cooking times and methods.
- Insulation:Understanding how heat flows in and out of buildings helps in designing effective insulation materials.
7. Aerospace and Mechanical Systems
- Rocket Propulsion:Thermodynamics is essential in designing engines that can convert chemical energy into the thrust needed for space travel.
- Aircraft Engines:Jet engines and turbines operate on thermodynamic cycles to convert fuel into mechanical work.
8. Pharmaceuticals and Healthcare
- Drug Design:Thermodynamics helps in understanding how drugs interact with biological molecules.
- Medical Devices:Devices like MRI machines and incubators operate based on the principles of thermodynamics.
You Might be Interested in These:

Comments
All Comments (0)
Join the conversation