Bohr’s Model of an Atom: Danish Physicist Niels Bohr received his Ph.D. from the University of Copenhagen in 1911. In 1922, he was awarded the Nobel Prize in Physics for his work on the atom’s structure. Let’s find out more about his work on Bohr's Model of an Atom.
What is Bohr's Model of an Atom?
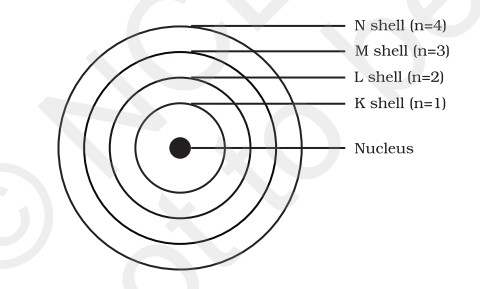
Scientist Niels Bohr introduced the Bohr’s model of an atom in 1913. It was introduced to bypass the objections that were put forward against the Rutherford model which stated that a positively charged nucleus is surrounded by electrons. Bohr's model of an atom stated that electrons move in fixed orbitals and that each orbit has a fixed energy. As per NCERT, ‘it was based on the postulate that only certain special orbits known as discrete orbits of electrons are allowed inside the atom and while revolving in discrete orbits the electrons do not radiate energy. These orbits or shells are energy levels and are represented by the letters K,L,M,N’.
Also Check: CBSE Science Video Courses for Class 12 Students
Postulates of Bohr’s Model of an Atom are:
The electron in the hydrogen atom can move around the nucleus in a circular path of fixed radius and energy. These paths are called orbits, stationary states or allowed energy states. These orbits are arranged concentrically around the nucleus.
The energy of an electron in the orbit does not change with time. However, the electron will move from a lower stationary state to a higher stationary state when required amount of energy is absorbed by the electron or energy is emitted when electron moves from higher stationary state to lower stationary state.The energy change does not take place in a continuous manner.
The frequency of radiation absorbed or emitted when transition occurs between two stationary states that differ in energy by ∆E, is given by
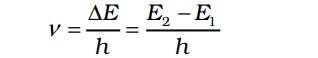
Where E1 and E2 are the energies of the lower and higher allowed energy states respectively. This expression is commonly known as Bohr’s frequency rule.
The angular momentum of an electron in a given stationary state can be expressed as an equation.
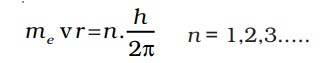
Limitations of Bohr’s Model of an Atom
NCERT has explained how Bohr’s model was too simple to account for the following points-
It fails to account for the finer details (doublet, that is two closely spaced lines) of the hydrogen atom spectrum observed by using sophisticated spectroscopic techniques. This model is also unable to explain the spectrum of atoms other than hydrogen, for example, helium atom which possesses only two electrons. Further, Bohr’s theory was also unable to explain the splitting of spectral lines in the presence of magnetic field (Zeeman effect) or an electric field (Stark effect).
It could not explain the ability of atoms to form molecules by chemical bonds.
Note: All images have been taken from NCERT Class 9 Science textbook.
Also, check
CBSE Class 9 Science Notes for Chapter 4 - Structure of the Atom
CBSE Class 11 Chemistry Notes: Structure of Atom (Part - II)
CBSE Class 12 Physics Atoms Formula List, Definitions, and Diagrams
Comments
All Comments (0)
Join the conversation