Lewis Structure of Carbon Dioxide: Carbon dioxide (CO2) is a fundamental molecule in chemistry, playing a crucial role in various biological and environmental processes. Understanding its Lewis structure, molecular geometry, hybridisation, and polarity helps in knowing its behaviour and interactions.
Who gave the Lewis Structure?
Lewis structures were introduced by American chemist Gilbert N. Lewis. He developed this notation system in 1916 to represent the valence electrons of atoms and to illustrate the bonding between atoms within a molecule. His work laid the foundation for modern theories of chemical bonding and molecular structure.
What are Lewis Structures?
Lewis structures, also known as Lewis dot diagrams, are diagrams that represent the bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule. They are used to show how valence electrons are arranged among atoms in a molecule. The key features include atoms, valence electrons, bonds, and lone pairs.
How to Draw the Lewis Structure for CO2?
- Determine the total number of valence electrons:
- Carbon (C) has 4 valence electrons.
- Each oxygen (O) has 6 valence electrons.
- Total = 4 + (6 x 2) = 16 valence electrons.
- Place the least electronegative atom in the centre:
- Carbon is less electronegative than oxygen, so carbon is the central atom.
- Distribute the electrons around the atoms:
- Place two electrons (a single bond) between carbon and each oxygen to form the initial connections.
- Distribute the remaining electrons to satisfy the octet rule for each atom.
- Complete the octets:
- Each oxygen needs 8 electrons to complete its octet. Initially, each oxygen gets 6 electrons in lone pairs, plus the 2 from the bond with carbon.
- Carbon will need to form double bonds with each oxygen to complete its octet.
- Finalise the structure:
- The Lewis structure of CO2will have double bonds between carbon and each oxygen, with no lone pairs on the carbon and two lone pairs on each oxygen.
Lewis Structure for CO2
The Lewis structure for CO2 is represented as:
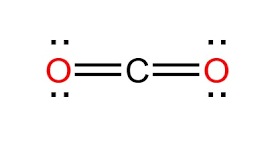
Each line represents a pair of shared electrons, forming double bonds between the carbon and each oxygen atom.
Molecular Geometry of CO2
The molecular geometry of CO2 is linear. This is due to the arrangement of the electron pairs around the central carbon atom. With two regions of electron density (the double bonds) and no lone pairs on the carbon atom, the bond angle between the oxygen atoms is 180 degrees, resulting in a linear shape.
Hybridisation of CO2
The hybridisation of the central carbon atom in CO2 is sp. This occurs because the carbon atom forms two sigma bonds with the oxygen atoms using sp hybrid orbitals, while the pi bonds are formed using unhybridised p orbitals. This sp hybridisation leads to a linear geometry.
Polarity of CO2
Despite having polar bonds (due to the difference in electronegativity between carbon and oxygen), CO2 is a nonpolar molecule. This is because the molecule is linear, and the dipole moments of the two C=O bonds cancel each other out, resulting in no overall dipole moment.
Understanding the Lewis structure and other properties of CO2 is is an essential part of chemistry, providing insights into molecular bonding, geometry, and behaviour of the carbon dioxide molecule.
References and Further Reading
You can refer to the reference material provided in the below-mentioned links for better preparation and understanding of scientific concepts.
- Books
NCERT Class 10 Science Textbook PDF
NCERT Class 12 Physics Textbook PDF
- Online Resources
Comments
All Comments (0)
Join the conversation