Sulfuric Acid is a strong acid and its corrosive in nature. It is also known as Mattling acid or Oil of vitriol. At high concentration levels, it acts as an oxidizing agent and dehydrating agent. Sulfuric acid has no colour and is odorless. The Sulfuric acid is water soluble and releases heat when mixed in water. It is primarily used by fertilizer manufacturers.
Sulfuric acid is a very strong acid, it ionizes completely to make hydronium ions and hydrogen sulfate ions. Hydrogen sulfate ions further ionise in a very diluted solution to give sulfate ions.
Formula of Sulfuric Acid
The chemical formula of Sulfuric acid is H2SO4. It's highly corrosive in nature with many industrial uses and commonly referred to as battery acid when diluted with water.

Read More: Hydroxide: Definition, Formula, Reactions And Examples!
Sulfuric Acid Structure – H2SO4
Here the Chemical and 3D structure of Sulfuric Acid for better understanding:
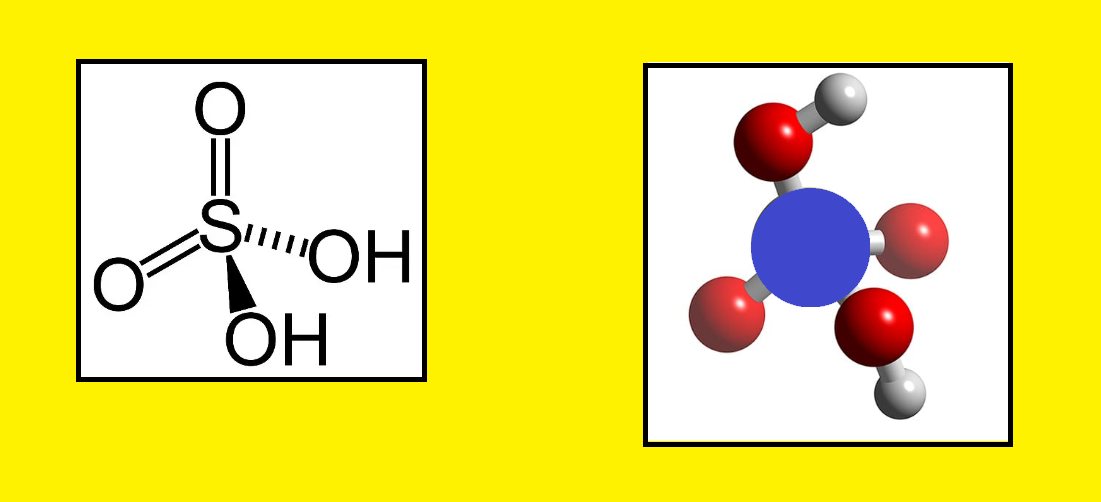
Read: Atomic Mass of Elements
Properties of Sulfuric Acid
Find below the properties of sulfuric acid in the tabular format for better understanding:
| Sulfuric Acid Formula | H2SO4 |
| Density | 1.84 g/cm³ |
| Boiling point | 337° C |
| Freezing Point | 10° C |
| Acidity | Sulfuric Acid is strong acid |
| Molar Mass | 98.079 g/mol |
Also Check:
- CBSE Class 11 Science Video Courses And Mock Tests By Subject Matter Experts
- CBSE Class 12 Science Video Courses And Mock Tests By Subject Matter Experts
Related:
Quantum Numbers: Principal Quantum Number, Azimuthal, Magnetic And Electron Spin Quantum Number!
Comments
All Comments (0)
Join the conversation