You must have heard about Mahatma Gandhi's Dandi March. Did you know that salt was such an important part in our struggle for freedom?
What is salt? Salt, also commonly known as table salt, is a refined, crystalline mineral compound primarily used in cooking, food preservation, and medical applications. Salt is produced in the process of reaction between an acid and a base. Like common salt, which is mined from underground or evaporated seawater, is then heavily processed to be super pure white.
Table salt is necessary for seasoning and enhancing the taste of food items and also for our bodies to function properly, such as maintaining fluid balance and sending nerve signals. Speaking of history, the use of salt was introduced by Europeans in some parts of the Western Hemisphere and India.
Seawater contains on average about three per cent salt, but high concentrations can range from 1 to 5 per cent. If the world's oceans dried, we would have approximately 4.5 million cubic miles of rock salt.
Let us now learn what the chemical name of table salt that we widely use in everyday life is. We will also explore the chemical formula and uses of sodium chloride.
What is the chemical name of table salt?
The chemical name of table salt is sodium chloride. It is colourless or white when pure. In its physical form, it is transparent to translucent cubic crystals, sometimes powder or granules. The molecular weight of sodium chloride is 58.44 g/mol.
What is the chemical formula of Table Salt?
Table salt is commonly known as sodium chloride, and its chemical formula is NaCl. The sodium and chloride ions are present in the ratio of 1:1. NaCl is water soluble and consists of sodium cation and chloride anion. The pH level of NaCl is 7.
Properties of Sodium Chloride
Below are the molecular weight, density, boiling point, and melting point of sodium chloride.
| NaCl | Sodium chloride |
| Molecular Weight/ Molar Mass of sodium chloride | 58.44 g/mol |
| Density of sodium chloride | 2.165 g/cm3 |
| Boiling Point of sodium chloride | 1.413 °C |
| Melting Point of sodium chloride | 801 °C |
Structure of Sodium Chloride
Table salt or sodium chloride ionic compound is formed when a sodium atom transfers one electron to a chlorine atom thus creating a positive charged sodium ions and negatively charged chloride ions that then attract each other. Industrially speaking, common salt is made from evaporating seawater or mined as rock salt (halite) and then refined in labs.
Below is the structure of sodium chloride:
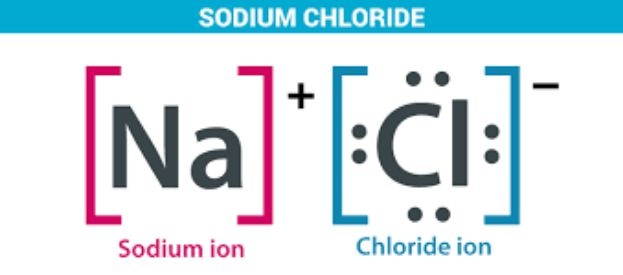
Uses of Sodium Chloride
Sodium Chloride is an important raw material for various materials in our daily lives. See below:
- Seasoning and culinary
- Food preservation
- Curing and marinating meats
- Saline solution (IV drips, nasal sprays, eye drops)
- Electrolyte balance and medicinal applications
- De-icing (spread on roads and sidewalks to lower the freezing point of water and ice)
- Cleaning products
- Fire extinguishers
- Shampoo and toothpastes
- Paper industry
- Textile industry
- Construction of roads
- Water softening
- Other industries like glass, rubber, plastics, oil and gas.
Comments
All Comments (0)
Join the conversation