Check unofficial CBSE Answer Key of Term 1 CBSE Class 12 Chemistry Question Paper 2021-22. Question paper of Term 1 CBSE Class 12 Chemistry question paper 2021-22 is also available here for download along with the answer key.
Disclaimer: This CBSE 12th Chemistry answer key 2021-22 is for reference purposes and is not final. It is expected that the Official CBSE Class 12 Chemistry answer key 2021-22 will be published by the board after the declaration of the CBSE result.
Latest CBSE Updates:
CBSE 12th Chemistry (Term 1) Board Exam 2021-22: Paper Analysis, Review, Answer Key & Latest Updates!
CBSE 12th Chemistry Term 1 Question Paper 2021-22 (Term 1) & Answer Key: Check Now
CBSE Answer Key: 12th Chemistry Term 1 Question Paper 2021-22 (Unofficial)
1. Which one of the following pairs will form an ideal solution?
(A) Chloroform and acetone
(B) Ethanol and acetone
(C) n-hexane and n-heptane
(D) Phenol and aniline
Answer: (C)
2. Which of the following is known as amorphous solid?
(A) Glass
(B) Plastic
(C) Rubber
(D) All of these
Answer 2: (A)
3. The structure of pyrosulphuric acid is
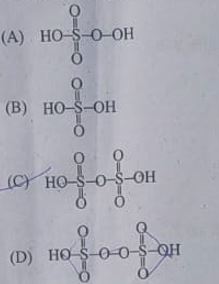
Answer 3: (C)
4. The C-O-H bond angle in alcohol is
(A)slightly greater than 109o28’.
(B)Slightly less than 109o28’.
(C)Slightly greater than 120o.
(D)Slightly less than 120o.
Answer: (B)
6. Nucleosides are composed of
(A) a pentose sugar and phosphoric acid
(B) a nitrogenous base and phosphoric acid
(C) a nitrogenous base and a pentose sugar
(D) a nitrogenous base, a pentose sugar and phosphoric acid
Answer: (C)
7. The oxidation state of -2 is most stable in:
(A) O
(B) S
(C) Se
(D) Te
8. Which of the following is not a characteristic of a crystalline solid?
(A) A true solid
(B) A regular arrangement of constituent particles
(C) Sharp melting point
(D) Isotropic in nature
9. Which of the following formula represents Raoult's law for a solution containing non-volatile solute?
(A) psolute = posolule. xsolute
(B) p = KH.X
(C) PTotal = PSolvent
(D) psolute = posolvent. Xsolvent
10. An azeotropic solution of two liquids has a boiling point lower than either of the two
when it (At shows a positive deviation from Raoult's law. (B) shows a negative deviation from Raoult's law. (C) shows no deviation from Raoult's law. (D) is saturated
11. Which of the following crystal will show metal excess defect due to extra cation?
(A) AgCl
(B) NaCl
(c) FeO
(d) ZnO
.
.
.
.
This is a developing story, more answer will be available here shortly.
Comments
All Comments (0)
Join the conversation