CBSE Class 12 Chemistry Chapter 8 Competency-Based Questions With Answer Key 2024-25: Are you also a CBSE board student looking to practise some questions for your board exam? Well, don't worry as we got you covered. Students can get the competency-based questions along with the answer key for Chemistry Chapter 8 Haloalkanes and Haloarenes and can also download the PDF for free.
Also, check
CBSE Class 12 Chemistry Chapter-8 Competency-Based Questions With Answer Key 2024-25
| Q.No | Question | Marks |
| Free Response Question/Subjective Type | ||
| Q.153 | 2-bromooctane reacts with alcoholic NaOH to give 2-octanol as shown below. 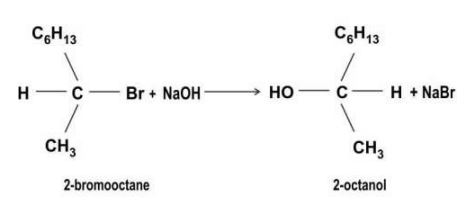 (a) Identify the type of substitution reaction mechanism. Justify your answer. (b) What effect will it have on the rate of the reaction if: (i) the concentration of NaOH is reduced by half? (ii) the concentration of 2-bromooctane is reduced by half? | 2 |
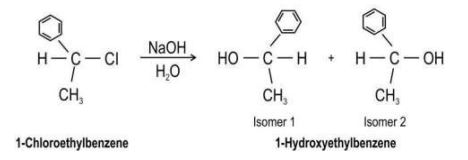
| Q.154 | 1-chloroethylbenzene undergoes hydrolysis by aqueous sodium hydroxide to give a mixture of two isomers as shown below. (a) State if the reaction follows the SN1 or SN2 mechanism. (b) Draw the structure of the intermediate formed in the reaction. (c) Explain why two isomers are formed and which one will predominate. (d) Compare the rate of hydrolysis of 1-chloroethylbenzene with that of 1- 1-bromoethylbenzene under similar conditions. Justify your answer. | 4 |
| Q.155 | (a) Which of the following two compounds has a chiral centre? 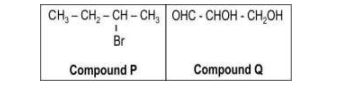 (b) Two compounds X and Y are enantiomers of each other. Name one physical property that: (i) is the same for X and Y. (ii) is different for X and Y. | 2 |
CBSE Class 12 Chemistry Chapter 8 Haloalkenes and Haloarenes Competency-Based Questions Answer Key 2024-25
| Q.No | Answers | Marks |
| Q.153 | (a) 0.5 marks for each of the following: - SN2 mechanism - The configuration of the product is opposite to that of the reactant. (b) 0.5 marks each for the following: (i) The rate of reaction will be reduced by half. (ii) The rate of reaction will be reduced by half. | 2 |
| Q.154 | (a) SN1 mechanism (b) (c) 0.5 marks for each of the following: - The intermediate carbonium ion has a planar structure. - The OH- ion can attack the intermediate either from the rear or from the front (side of departing Cl- ion) - Isomer 1 will predominate. - The departing Cl- ion shields the front side from attack by the OH- nucleophile. (d) 0.5 marks for each of the following: - The rate of reaction would be faster with 1-bromoethylbenzene. - The bromonium ion Br- is a more stable leaving group as it is larger than the Cl- ion and the charge is spread over a larger area. | 4 |
| Q.155 | (a) Both, compound P and compound Q have a chiral centre. (b) (i) 0.5 marks each for any one example such as: - melting point - boiling point - refractive index (ii) direction of rotation of the plane of polarized light [0.5 marks] | 2 |
Now, that the students have got the questions along with the answer key for CBSE Class 12 Chemistry Chapter-8 Haloalkenes and Haloarenes, we are also providing a free pdf to download the question-answer key for free. Check the link below.
Other Related Links
Comments
All Comments (0)
Join the conversation