Key Points
- Provides solutions for NCERT in-text and exercise questions for Class 10 Science Chapter 4.
- Covers topics like electron dot structures, isomers, and properties of carbon.
- Explains homologous series, distinguishing between saturated and unsaturated hydrocarbons, and the cleaning action of soaps.
NCERT Solutions For Class 10 Chapter Carbon And Its Compounds: Well, NCERT books are considered to be the best resource for building a solid foundation in theoretical concepts and strengthening basics. The in-text and end-of-chapter questions in NCERT textbooks are important for the students to prepare for the exam and score well. This also helps the students to clear their concepts and deepen their understanding of particular chapters or topics. It is always recommended to refer to NCERT textbooks for the preparation of the exam.
In this article, students can get comprehensive solutions for all the NCERT in-text and final exercise questions from CBSE Class 10 Science Chapter 4, Carbon and its Compounds. All the NCERT solutions have been compiled into a PDF format, available for free download to students.
| Also, check |
NCERT Solutions For Science Class 10 Chapter 4 Carbon And Its Compounds:
Intext Solution Page No - 61
1. What would be the electron dot structure of carbon dioxide which has the formula CO2?

2. What would be the electron dot structure of a molecule of sulphur which is made up of eight atoms of sulphur? (Hint – The eight atoms of sulphur are joined together in the form of a ring.)
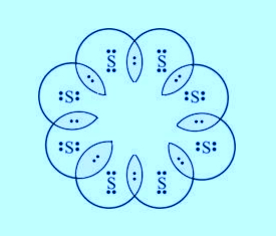
Intext Solution Page No - 68-69
1. How many structural isomers can you draw for pentane?
The structural isomers of pentane are as follows:
n-pentane
.jpg)
2-methyl butane 2
.jpg)
2-dimethylpropane
.jpg)
2. What are the two properties of carbon which lead to the huge number of carbon compounds we see around us?
Two properties of carbon which lead to the huge number of carbon compounds we see around us are given below:
- Carbon has six valence electrons which is a high number of valency.
- Covalent bonding happens easily with carbon atoms and numerous others, such as oxygen, chlorine, nitrogen, sulphur, hydrogen, etc.
3. What will be the formula and electron dot structure of cyclopentane?
The formula and electron dot structure of cyclopentane are given below:
.jpg)
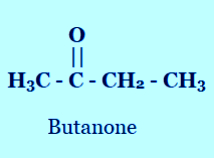
4. Draw the structures for the following compounds. (i) Ethanoic acid (ii) Bromopentane (iii) Butanone (iv) Hexanal.
(i) Ethanoic acid
.jpg)
(ii) Bromopentane
.jpg)
(iii) Butanone
(iv) Hexanal
.jpg)
5. How would you name the following compounds?
(i) Bromoethane
(ii) Methanal or Formaldehyde
(iii)1 – Hexyne
Intext Solutions Page No - 71
1. Why is the conversion of ethanol to ethanoic acid an oxidation reaction?
.jpg)
The conversion of ethanol to ethanoic acid involves the removal of the hydrogen atom and the addition of oxygen, and it is an oxidation reaction. In the first step, an H2 molecule is removed from ethanol to form ethanal. As the loss of hydrogen is oxidation, the reaction is an oxidation reaction. Similarly, an oxygen atom is added to form ethanoic acid from ethanol. As the gain of oxygen is called oxidation, the reaction is an oxidation reaction.
2. A mixture of oxygen and ethyne is burnt for welding. Can you tell why a mixture of ethyne and air is not used?
A mixture of oxygen and ethyne is burnt for welding instead of a mixture of ethyne and air because the production of heat is very important for welding metals. When oxygen and ethyne are burnt, it burns completely and produces a higher temperature than air and ethyne. Oxygen and ethyne produce a very hot blue flame, but the mixture of air and ethyne gives out a sooty flame which means that there are unburnt particles, resulting in lesser heat.
Intext Solution Page No - 74
1. How would you distinguish experimentally between an alcohol and a carboxylic acid?
Solution: In reaction with sodium carbonate, carboxylic acids produce carbon dioxide gas which turns lime water milky, whereas alcohols do not give this reaction. This experiment can be used to distinguish alcohol and carboxylic acid. The reaction of the carboxylic acid with sodium carbonate: 2CH3COOH + Na2CO3 → 2CH3COONa + H2O + CO2
2. What are oxidising agents?
Solution: Oxidising agents are those compounds which either remove hydrogen or add oxygen to a compound. For example, halogens, potassium nitrate, and nitric acid.
Intext Solution Page No - 76
1. Would you be able to check if water is hard by using a detergent?
It is impossible to check if water is hard using a detergent because detergents are salts of ammonium or sulphonates of long-chain carboxylic acids. Unlike soaps, they do not react with calcium and magnesium to distinguish the nature of water.
2. People use a variety of methods to wash clothes. Usually, after adding the soap, they ‘beat’ the clothes on a stone, or beat it with a paddle, scrub with a brush or the mixture in a washing machine. Why is agitation necessary to get clean clothes?
Agitation is necessary to get clean clothes as it aids soap micelles to trap the oil, grease or any other impurities that have to be removed. When they are being beaten or agitated, the particles are removed from the clothes’ surfaces and go into the water, thus cleaning the clothes.
NCERT Exercises Page No. - 77-78
1. Ethane, with the molecular formula C2H6 has
(a) 6 covalent bonds
(b) 7 covalent bonds
(c) 8 covalent bonds
(d) 9 covalent bonds.
Solution: (b) Ethane has 7 covalent bonds.
2. Butanone is a four-carbon compound with the functional group
(a) carboxylic acid
(b) aldehyde
(c) ketone
(d) alcohol
Solution: (c) The functional group of butanone is ketone.
3. While cooking, if the bottom of the vessel is getting blackened on the outside, it means that
(a) the food is not cooked completely
(b) the fuel is not burning completely
(c) the fuel is wet
(d) the fuel is burning completely
Solution: (b)While cooking, if the bottom of the vessel is getting blackened on the outside, then it means that the fuel is not burning completely.
4. Explain the nature of the covalent bond using the bond formation in CH3Cl.
Solution: Carbon forms a bond by sharing its four electrons with other atoms. These bonds that are formed by sharing electrons are known as covalent bonds. In this bonding, both atoms share the valence electrons. Here, carbon requires 4 electrons to complete its octet and each hydrogen atom requires one electron to complete its octet. Chlorine requires one electron to complete the octet. Thus, all of these atoms share the electrons. With this formation, carbon forms 3 bonds with hydrogen and one bond with chlorine.
.jpg)
5. Draw the electron dot structures for
(a) ethanoic acid
(b) H2S
(c) propanone
(d) F2
Solutions: (a) ethanoic acid
.jpg)
(b) H2S
.jpg)
(c) Propanone
.jpg)
(d) F2
.jpg)
6. What is a homologous series? Explain with an example.
Solution: A homologous series is a group of organic compounds with a similar general formula, where each successive member differs by a CH₂ unit. Compounds in a homologous series exhibit similar chemical properties but have gradually changing physical properties (e.g., boiling points) due to increasing molecular size. Each member has the same functional group, leading to predictable reactivity.
Example: The alkane series (CnH2n+2) is homologous. It includes methane (CH₄), ethane (C₂H₆), propane (C₃H₈), and so on, where each compound differs by one CH₂ group, showing similar chemical behaviour like combustion.
7. How can ethanol and ethanoic acid be differentiated based on their physical and chemical properties?
| Property | Ethanol (C₂H₅OH) |
Ethanoic Acid (CH₃COOH) |
| Physical State | Colourless liquid | Colourless liquid |
| Odor | Mild, pleasant odour (alcohol-like) |
Sharp, vinegar-like odour |
| Taste | Mild, burning taste |
Sour, acidic taste |
| Boiling Point | 78.37°C | 118.1°C |
| Solubility in Water | Completely soluble |
Completely soluble |
| pH | Neutral to slightly acidic (~7) | Acidic (~2.4) |
| Reaction with Sodium | Reacts to form sodium ethoxide and hydrogen gas |
Reacts to form sodium ethanoate and hydrogen gas |
| Reaction with Carbonates | No reaction with sodium bicarbonate or carbonates |
Reacts with sodium bicarbonate/carbonates to release carbon dioxide gas |
| Reaction with Litmus | No change (neutral solution) |
Turns blue litmus red (acidic) |
| Ester Formation | Reacts with acids to form esters |
Reacts with alcohol to form esters |
| Flammability | Highly flammable |
Less flammable than ethanol |
8. Why does micelle formation take place when soap is added to water? Will a micelle be formed in other solvents such as ethanol?
A soap is a sodium or potassium salt of long-chain fatty acids. It consists of polar and non-polar ends. The polar end is hydrophilic while the non-polar end is hydrophobic. When soap is added to water, soap molecules arrange themselves in a cluster. In this arrangement, they keep the non-polar portion out of water and the polar ends are on the surface of the cluster. The dirt present on clothes is insoluble in water. Thus, the hydrophobic ends of the clusters attach themselves to the dirt. This cluster formation in which the dirt is entrapped is called micelle. Micelle formation does not take place in alcohol because the alkyl chain of soap becomes soluble in alcohol.
9. Why are carbon and its compounds used as fuels for most applications?
Solution: Carbon and its compounds are used as fuels for most applications because they have high calorific values and give out a lot of energy. Most of the carbon compounds give a lot of heat and light when burnt in the air.
10. Explain the formation of scum when hard water is treated with soap.
Solution: Scum is produced from the reaction of hard water with soap. Calcium and magnesium present in the hard water form an insoluble precipitate called scum.
11. What change will you observe if you test soap with litmus paper (red and blue)?
Solution: When soap is dissolved in water due to the formation of alkaline NaOH or KOH, the solution is alkaline. The solution changes the colour of the red litmus to blue, but in the soap solution, the blue litmus remains blue.
12. What is hydrogenation? What is its industrial application?
Solution: Hydrogenation is a process or a chemical reaction between hydrogen and other compounds. It is usually done in the presence of catalysts. For example, nickel, palladium or platinum. Hydrogenation is used mainly to saturate organic compounds.
13. Which of the following hydrocarbons undergo addition reactions: C2H6, C3H8, C3H6, C2H2 and CH4?
Solution: Unsaturated hydrocarbons undergo additional reactions. C3H6 and C2H2 are unsaturated hydrocarbons which undergo additional reactions.
14. Give a test that can be used to differentiate between saturated and unsaturated hydrocarbons.
Solution: The bromine water test is used to differentiate between the unsaturated compounds (like alkenes and alkynes) and the saturated compounds. For this purpose, bromine is used in the form of bromine water. A solution of bromine in water is called bromine water. Bromine water has a red-brown colour due to the presence of bromine in it. When bromine water is added to an unsaturated compound, then bromine gets added to the unsaturated compound, and the red-brown colour of bromine water is discharged. So, if an organic compound decolourises bromine water, then it will be an unsaturated hydrocarbon (containing a double bond or a triple bond), but saturated hydrocarbons (alkanes) do not decolourise bromine water. The bromine water test is performed to differentiate between the unsaturated compounds (like alkenes and alkynes) and the saturated compounds. When bromine water is added to an unsaturated hydrocarbon, the red-brown colour of the bromine solution is discharged. So, if there is dis-colouration, then the compound will be an unsaturated hydrocarbon.
15. Explain the mechanism of the cleaning action of soaps.
Solution: There are so many impurities and dirt mixed in water, and most of all, the dirt does not dissolve in the water. Soap molecules are a combination of salts such as sodium or potassium. The molecules are of a long chain of carboxylic acids. So, when the carbon chain is dissolved in oil and the ionic end is dissolved in the water, the soap starts cleansing and trapping the dirt. When this happens, the soap molecules form structures called micelles that are used for capturing the oil droplets, and then the other end is the ionic faces. This will then form an emulsion in water and help in dissolving the dirt or impurities when the clothes are washed. The soap molecules have different properties at different ends. The first end is the hydrophilic end, which dissolves in the water and is attracted towards the water, and the second one is the hydrophobic end, which is dissolved in the hydrocarbons and is repulsive to water. The hydrophobic tail aligns itself along the surface of the water because it is not soluble in the water.
We are also providing the free PDF to download the NCERT Solutions For Class 10 Chapter 4: Carbon And Its Compounds for free. Check the link below.
CHECK: NCERT Solutions For Class 10 Chapter 4: Carbon And Its Compounds Free PDF Download |
Other Related Links
- NCERT Books for Class 10 All Subjects PDF (2024-25)
- CBSE Class 10 Exam Pattern 2024-2025
- CBSE Class 10 Pre-Board Sample Paper 2025
- CBSE Class 10 Syllabus 2024-25
- CBSE Class 10 Subject-Wise 90 Days Study Plan for Board Exam 2025
Comments
All Comments (0)
Join the conversation