NCERT Solutions for Class 12 Biomolecules Chapter 10: Both intext and exercise questions are important for students from the perspective of 2025 examination. Solving these questions boosts the confidence of students and prepares them for the Board examination. Chemistry is a subject that may look tough but with the right approach, students can tackle the questions easily. To help students, we have provided the NCERT Solutions for Class 12 Chemistry Chapter 10 Biomolecules here.
NCERT solutions are the best study material to crack board exams. These solutions help students to develop a clear understanding of subjects. They have been prepared by subject experts to enable students to find the right strategy to handle problems given in textbook.
Also Read:
| CBSE Class 12 Chemistry Exam Pattern and Marking scheme 2024-25 |
Continue reading to download the NCERT Solutions for Class 12 Chemistry Chapter 10 Biomolecules from the direct link given in this article. The solutions have been given in PDF format so students can save and view the PDFs on phones, tablets, computers and laptops.
NCERT Class 12 Chemistry Chapter 10 Biomolecules Solutions
Students check the questions and solutions for intext and exercise questions from NCERT Class 12 Chapter 10 Biomolecules carefully.
Intext Questions and Solutions Page no. 290
10.1 Glucose or sucrose are soluble in water but cyclohexane or benzene (simple six membered ring compounds) are insoluble in water. Explain.
Sol. The solubility of a compound is depicted by the presence of H-bonding. Glucose has 5 and sucrose has 8 -OH groups. These -OH groups can form H-bonding with water easily and thus are soluble. Whereas benzene and cyclohexane do not have -OH bonds and thus cannot form bonds with water. Therefore they are not soluble.
10.2 What are the expected products of hydrolysis of lactose?
Sol. Lactose contains β--D-galactose and β--D-glucose which on hydrolysis generates the same compounds.
10.3 How do you explain the absence of aldehyde group in the pentaacetate of D-glucose?
Sol. The structure of D-glucose reacts with hydroxylamine to lead to the formation of an oxime due to the presence of aldehydic group in the structure. Whereas the pentaacetate of D-glucose is not an open structure and there is no reaction with hydroxylamine. This explains the absence of aldehydic group in the pentaacetate of D-glucose.
Intext Questions and Solutions Page no. 295
10.4 The melting points and solubility in water of amino acids are generally higher than that of the corresponding halo acids. Explain.
Sol. The amino acid molecules have acidic as well as basic groups within them. Due to their dipolar nature, they give rise to zwitter ion when dissolved in water. On the other hand, halo acids are not dipolar. In this case, the zwitter ion is formed when the carboxyl group loses a proton. Thus the melting points and solubility in water of amino acids are generally higher than that of the corresponding halo acids.
10.5 Where does the water present in the egg go after boiling the egg?
Sol. When an egg is boiled, the proteins undergo denaturation and then goes under coagulation. The water present in excess is absorbed by the coagulated protein through H-bonding.
Intext Questions and Solutions Page no. 301
10.6 Why cannot vitamin C be stored in our body?
Sol. It is not possible to store vitamin C in our body as it is water soluble and thus cannot be retained due to constant urination.
10.7 What products would be formed when a nucleotide from DNA containing thymine is hydrolysed?
Sol. The hydrolysis of a nucleotide from DNA containing thymine would form two-deoxyribose and phosphoric acid.
10.8 When RNA is hydrolysed, there is no relationship among the quantities of different bases obtained. What does this fact suggest about the structure of RNA?
Sol. A DNA molecule has a double stranded structure in which adenine pairs with thymine and cytosine pairs with guanine. Upon hydrolysis, the amount of adenine produced is equal to that of thymine, while the amount of cytosine is equal to that of guanine. But upon hydrolysis of RNA, no such relationship between products comes up. Thus RNA has a single strand.
NCERT Class 12 Chemistry Chapter 10 Biomolecules Exercise Solutions
10.1 What are monosaccharides?
Sol. These are the simpler carbohydrates which cannot be hydrolysed further to its constituent aldehyde or ketone. Classification of monosaccharides on the basis of number of carbon atoms and the functional group:
a) Monosaccharide containing an aldehyde group are known as aldoses & those containing a keto group are known as ketoses.
b) On the basis of the number of carbon atoms Monosaccharides contain, they are further classified as trioses, tetroses, pentoses, hexoses, and heptoses.
10.2 What are reducing sugars?
Sol. Reducing sugars are carbohydrates which can reduce Fehling’s solution and Tollen’s reagent. Except sucrose, all monosaccharides and disaccharides are reducing sugars.
10.3 Write two main functions of carbohydrates in plants.
Sol. Two main functions of carbohydrates in plants are:
1. Cellulose is used to build the cell wall.
2. Starch acts as storage molecules.
10.4 Classify the following into monosaccharides and disaccharides. Ribose, 2-deoxyribose, maltose, galactose, fructose and lactose.
Sol. Monosaccharide: Ribose, 2-deoxyribose, galactose, fructose
Disaccharides: Maltose, lactose
10.5 What do you understand by the term glycosidic linkage?
Sol. Glycosidic linkage: It is the linkage formed between two monosaccharide units through an oxygen atom with the loss of a water molecule. For example: Sucrose molecule.
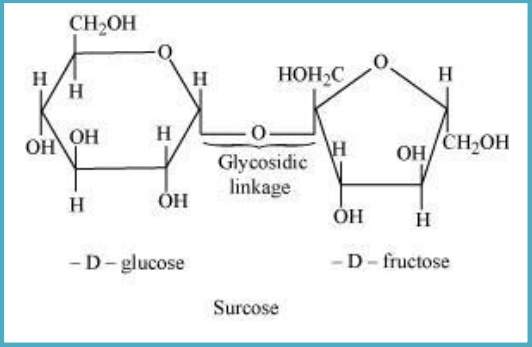
10.6 What is glycogen? How is it different from starch?
Sol. Both glycogen & starch are carbohydrates. Starch is composed of two components − amylose (15 − 20%) and amylopectin (80 − 85%).
Glycogen is a polysaccharide & is present in animals. In animals, carbohydrates are stored as glycogen. It consists of only one component whose structure is similar to amylopectin. Glycogen is more branched than amylopectin.
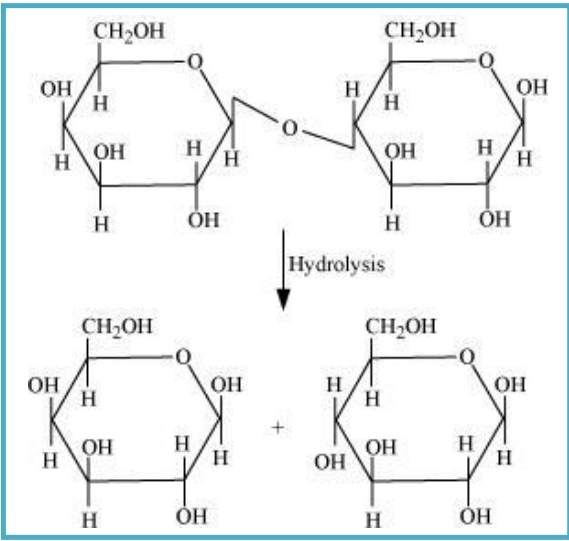
10.7 What are the hydrolysis products of (i) sucrose and (ii) lactose?
Sol. (i) On hydrolysis, sucrose gives one molecule of ∝-D glucose and one molecule of β- D-fructose.
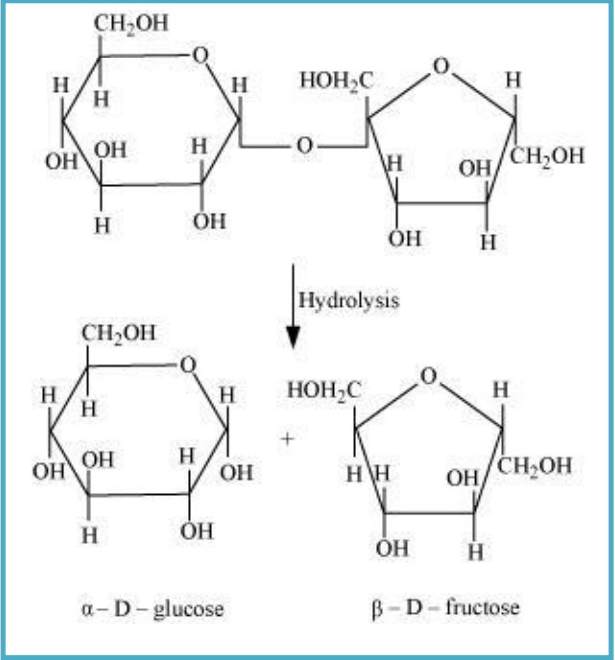
(ii) The hydrolysis of lactose gives β-D-galactose and β-D-glucose.
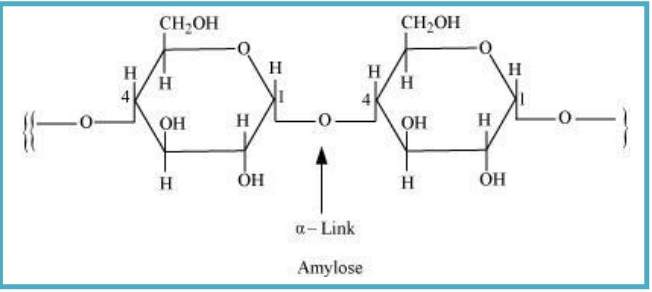
10.8 What is the basic structural difference between starch and cellulose?
Sol. Starch: It consists of two components − amylose and amylopectin.
Amylose is a long linear chain of ∝−D−(+)−glucose units which joins at C1 & C4 position, forming glycosidic linkage (∝-link).
Amylopectin is a branched-chain polymer of ∝-D-glucose units. In this, chain is further extended by C1 & C4 position, forming glycosidic linkage and the branching takes place at C1 & C6 position.
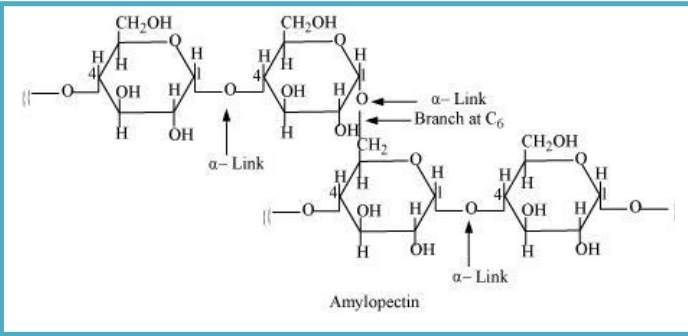
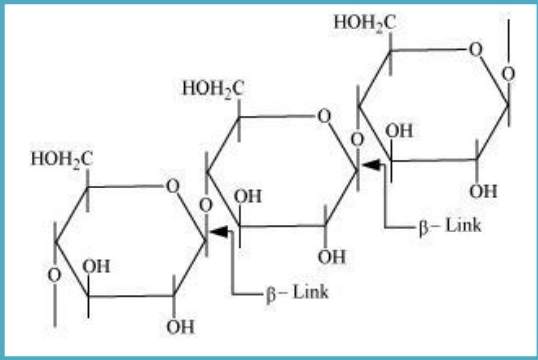
Cellulose: It is a straight-chain polysaccharide of β-D-glucose units which joins at C1−C4 & forms glycosidic linkage (β-link).
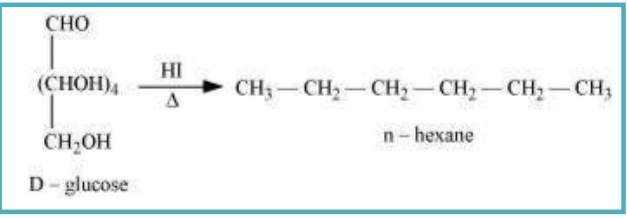
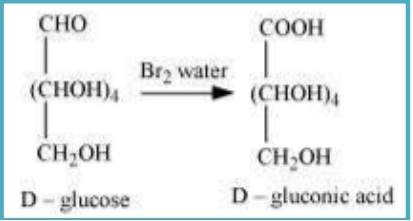
10.9 What happens when D-glucose is treated with the following reagents? (i) HI (ii) Bromine water (iii) HNO₃
Sol. (i) When D-glucose is heated with HI for long time, n-hxane is formed.
(ii) When D-glucose is treated with Br water, D- gluconic acid is formed.
(iii) On being treated with HNO₃, D-glucose gets oxidised to give saccharic acid.
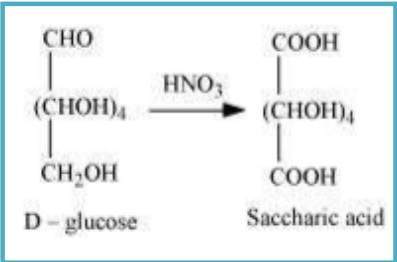
10.10 Enumerate the reactions of D-glucose which cannot be explained by its open chain structure.
Sol. Glucose exists in two crystalline forms − ∝ and β. The ∝-form crystallises from a concentrated solution of glucose at 303 K and the β-form crystallises from a hot and saturated aqueous solution at 371 K. This behaviour cannot be explained by the open chain structure of glucose.
a) Aldehydes give Schiff’s test, 2, 4-DNP test.
Glucose does not undergo these reactions.
b) Aldehydes react with NaHSO4 to form the hydrogen sulphite addition product.
Glucose does not undergo these reactions.
c) The pentaacetate of glucose does not react with hydroxylamine. This indicates that a free −CHO group is not present in glucose.
10.11 What are essential and non-essential amino acids? Give two examples of each type.
Sol. Essential amino acids: These amino acids are essential for the human body, but they cannot be synthesised in the body. Thus, their requirement should be completed through food. For Example: Leucine & Valine.
Non-essential amino acids: These amino acids can be synthesised in the body. They are also important for the human body. For example: Alanine & Glycine.
10.12 Define the following as related to proteins (i) Peptide linkage (ii) Primary structure (iii) Denaturation.
Sol. (i) Peptide linkage: When −COOH group of one molecule of an amino acid and −NH₂ group of another molecule of the amino acid are reacted, amide is formed. In this reaction, elimination of a water molecule takes place. This linkage is known as a peptide linkage.
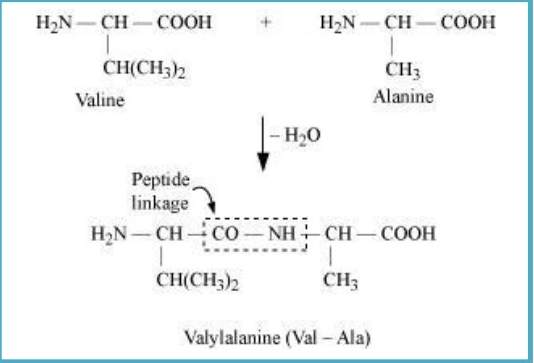
(ii) Primary structure: It is a specific sequence of linkages between amino acids in a polypeptide chain. Different sequences are present in different proteins. Any change in the sequence creates a different protein.
(iii) Denaturation: Protein has is a unique 3-dimensional structure and a unique biological activity. In such condition, it is active & takes part in reactions, & is known as native protein. However, when the native protein is subjected to physical changes such as change in temperature or chemical changes such as its H Bonds are disturbed, change in pH. These activities disturb the structure of protein. As a result, unfolding of the globules takes place which uncoils the helix. In this form, the protein loses its biological activity. This loss of biological activity by the protein is known as denaturation.
Denaturation destroys the secondary and the tertiary structures of the protein, but it does not alter the primary structure. For example: Coagulation of egg white when an egg is boiled.
10.13 What are the common types of secondary structure of proteins?
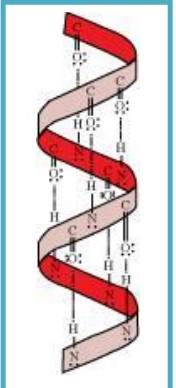
Sol. Two common types of secondary structure of proteins: - (i) ∝-helix structure (ii) β-pleated sheet structure.
∝- Helix structure: In this structure, the −NH group of an amino acid forms hydrogen bond with the –CO– group of the adjacent turn of the right-handed screw (∝-helix).
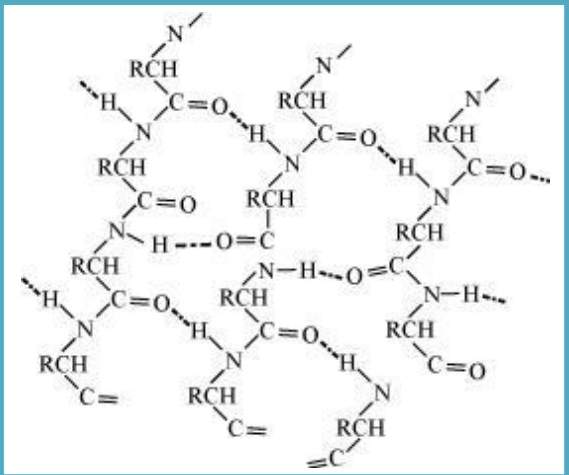
β- Pleated sheet structure: In this structure, all the peptide chains are stretched out to the maximum extension possible and then lay side by side. These peptide chains are held together by intermolecular hydrogen bonds. This structure looks like the pleated folds of drapery. Thus, it is known as βpleated sheet structure.
10.14 What type of bonding helps in stabilising the ∝-helix structure of proteins?
Sol. The hydrogen bonds formed between the −NH group of each amino acid & the -CO- group of the adjacent turns of the ∝-helix help in stabilising the helix.
10.15 Differentiate between globular and fibrous proteins.
Sol. Fibrous protein
It has a fibre-like structure. These proteins are held together by strong hydrogen and disulphide bonds.
They are generally responsible for structural purposes. For example, myosin is present in muscles; keratin is present in nails and hair.
It is insoluble in water.
Globular protein
In this protein, polypeptide chain is folded around itself, results in a spherical structure.
All enzymes come in this category of proteins. Some hormones are also globular proteins for example- insulin.
It is soluble in water.
10.16 How do you explain the amphoteric behaviour of amino acids?
Sol. In aqueous solution, amino acid forms zwitter ion. Carboxyl group of an amino acid can lose a proton and the amino group can accept a proton, forms a dipolar ion known as zwitter ion. In zwitter ionic form, amino acids can act both as an acid and base.
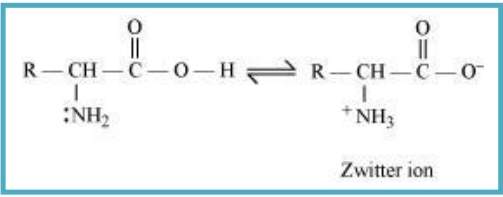
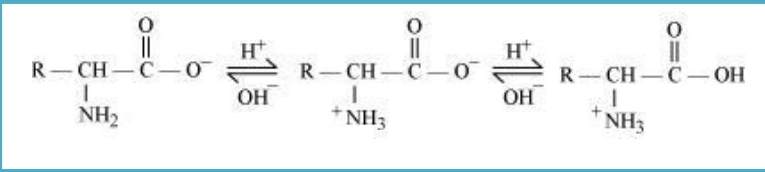
Hence, we can say that, amino acids show amphoteric behaviour.
10.17 What are enzymes?
Sol. Enzymes: They are proteins which catalyse biological reactions. They are very specific in nature and catalyse only a particular reaction for a particular substrate.
They are usually named after the particular substrate or class of substrate and sometimes after the particular reaction. For example: The enzyme used to catalyse the hydrolysis of maltose into glucose is named after sugar maltose, as maltase.
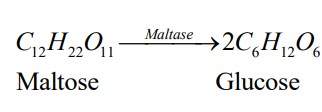
10.18 What is the effect of denaturation on the structure of proteins?
Sol. Denaturation destroys the secondary and the tertiary structures of the protein, but it does not alter the primary structure. During denaturation, secondary and tertiary-structured proteins get converted into primary-structured proteins. When their secondary and tertiary structures are destroyed, the enzyme loses its activity.
10.19 How are vitamins classified? Name the vitamin responsible for the coagulation of blood.
Sol. On the basis of their solubility in water or fat, vitamins are classified into two groups.
(i) Fat-soluble vitamins: - Vitamins which are soluble in fat and oils, but not in water, are known as fat-soluble vitamins. For example- Vitamins A, D, E, and K.
(ii) Water-soluble vitamins: Vitamins which are soluble in water are known as water-soluble vitamins. For example- vitamin B & C.
Vitamin K is responsible for the coagulation of blood.
10.20 Why are vitamin A and vitamin C essential to us? Give their important sources.
Sol. Vitamin A- Xerophthalmia (hardening of the cornea of the eye). It leads to night blindness.
Vitamin C - scurvy (bleeding gums)
Thus, we can say that, vitamin A & C are essential to us. The sources of vitamin A & C are:
Vitamin A- fish liver oil, carrots, butter, and milk
Vitamin C- citrus fruits, amla, and green leafy vegetables.
10.21 What are nucleic acids? Mention their two important functions.
Sol. Nucleic acids: These are the bio molecules found in the chromosomes of all living cells. These are also known as polynucleotide as they are long-chain polymers of nucleotides.
There are mainly two types of nucleic acids – DNA & RNA.
Two main functions of nucleic acids are:
(i) DNA is responsible for hereditary process.
(ii) DNA & RNA are responsible for protein synthesis in a cell.
10.22 What is the difference between a nucleoside and a nucleotide?
Sol. In nucleoside, base is attached to sugar at position.
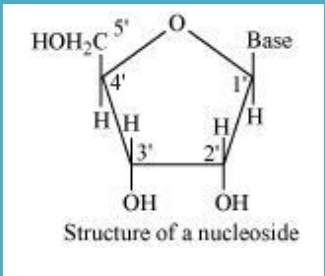
On the other hand, in a nucleotide, all the three basic components of nucleic acids (i.e., pentose sugar, phosphoric acid, and base) are present.
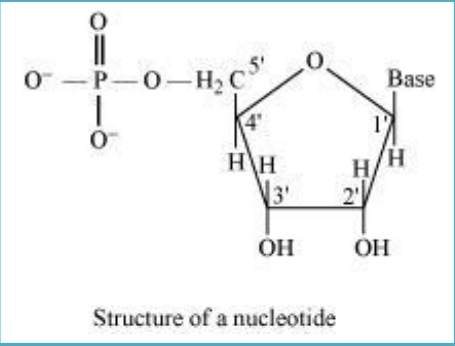
Nucleoside = Sugar + Base
Nucleotide = Sugar + Base + Phosphoric acid
10.23 The two strands in DNA are not identical but are complementary. Explain.
Sol. DNA is present in a helical structure, in which two strands are held together by hydrogen bonds between specific pairs of bases. Bases present on strands do not form bonds with same bases like Cytosine forms hydrogen bond with guanine, while adenine forms hydrogen bond with thymine. Thus, we can say that, two strands are complementary to each other. So that bases can form bonds.
10.24 Write the important structural and functional differences between DNA and RNA.
Sol. The structural differences between DNA and RNA are: -
DNA
The sugar molecule in DNA molecules is β-D-2 deoxyribose.
Here thymine (T) is present instead of uracil (U).
The helical structure of DNA is double-stranded.
RNA
The sugar molecule in RNA molecules is β-D-ribose.
Here uracil (U) is present & thymine (T) is absent.
The helical structure of RNA is single-stranded.
The functional differences between DNA and RNA are: -
DNA
DNA forms the chemical basis of heredity.
It does not synthesise proteins, but transfer coded messages for the synthesis of proteins in the cells.
RNA
RNA is not responsible for heredity.
It takes part in protein synthesis.
10.25 What are the different types of RNA found in the cell?
Sol. (i) Messenger RNA (m-RNA)
(ii) Ribosomal RNA (r-RNA)
(iii)Transfer RNA (t-RNA)
To download these solutions in pdf, refer to the below link:
| Download NCERT Class 12 Chemistry Chapter 10 Biomolecules Solutions PDF |
Also, check
- CBSE 12th Date Sheet 2025 Download PDF
- CBSE Class 12 Chemistry Competency-Based Questions With Answer Key 2024-25
- CBSE Class 12 Chemistry Topper Answer Sheet for 2025 Exams
- CBSE Class 12 Chemistry 30 Days Study Plan for 2025 Exam
- CBSE Class 12 Chemistry Previous Year Question Papers with Solutions PDF Download
- NCERT Solutions for Class 12 (2024-2025) All Subjects
- NCERT Books for CBSE Class 12 - Latest Edition
- NCERT Rationalised Content for Class 12
Related
How to Convert Celsius to Kelvin: Concept, Formula, and Major Differences
Comments
All Comments (0)
Join the conversation