Key Points
- CBSE Class 10 Science Exam is on February 20, 2025.
- 80 marks for theory, 20 for internal assessment.
- Sample papers and solutions available for download.
CBSE Class 10th SCIENCE Board Exam 2025: The CBSE Class 10 Science exam is scheduled for February 20, 2025 (Thursday). As an important subject, it plays a major role in boosting overall board exam scores. To help students prepare effectively, we provide the CBSE Class 10 Science Sample Paper with Solutions. Designed by experts, this resource is ideal for last-minute revision and practising important questions. The solutions offer clarity on correct answers. Download the CBSE Class 10 Science Paper PDF from the links below to strengthen your preparation and score well in the exam.
Important*
Features of CBSE Class 10 Science Sample Paper 2025
- Follows Latest Exam Pattern – The sample paper is designed as per the latest CBSE exam pattern to help students understand the question format.
- It includes All Question Types, including objective, short–answer, and long–answer questions, covering all important topics.
- Covers Full Syllabus – The sample paper includes questions from all chapters, ensuring complete syllabus revision.
- Provides Marking Scheme – Along with the paper, CBSE also provides a marking scheme to help students know how answers should be written.
- Boosts Exam Confidence – Practicing the sample paper helps students with time management and improves their confidence before the actual exam.
Check| CBSE Class 10th Science Syllabus 2024-25
CBSE Class 10 Science Sample Paper 2025 with Solutions
Check and download the Class 10 Science Sample Paper with Solutions to boost your preparation for the upcoming CBSE Class 10 Science Exam 2025:
SECTION- A
Section-A Select and write the most appropriate option out of the four options given for each of the questions 1 - 20.
1. Pb + CuCl2→ PbCl2 + Cu
The above reaction is an example of:
(a) Combination
(b) Decomposition
(c) double displacement
(d) displacement
2. Identify the non-metal which is lustrous.
(a) Sulphur
(b) Oxygen
(c) Nitrogen
(d) Iodine
3. The complete oxidation of ethanol gives
(a) Acetic acid/ethanoic acid
(b) CO2 and water
(c) Ethanal
(d) Acetone/Ethanone
4. Which of the following is not a chemical reaction?
(a) Souring of milk
(b) Dissolution of sugar in water
(c) Rusting of iron
(d) Digestion of food
5. Identify the totally impossible outcome of Mendel’s Experiment (cross-breeding purebred tall and short pea plants)
(a) 3 tall 1 short plant
(b) 24 tall and 8 short plants
(c) 8 tall and 0 short plants
(d) 4 tall plants and 1 medium-height plant
IMPORTANT:
- CBSE Class 10 Science Sample Paper For Board Exams 2025 (By CBSE Board)
- CBSE Class 10th Science Exam Pattern 2025
6. Who have a perfect pair of sex chromosomes?
(a) Girls only
(b) Boys only
(c) Both girls and boys
(d) It depends on many other factors
7. Every food chain in the ecosystem begins with.......... which is the original source of food.
(a) Saprophytes
(b) Parasites
(c) Producers
(d) Herbivores
8. Which property of metals is used for making bells and strings of musical instruments like Sitar and Violin?
(a) Sonorousness
(b) Malleability
(c) Ductility
(d) Conductivity
9. The number of isomers of pentane is 1
(a) 2
(b) 3
(c) 4
(d) 5
10. Name the substances that build up in the muscles during vigorous physical exercise and may cause cramps.
(a) Ethanol + Carbon dioxide + Energy
(b) Lactic acid + Energy
(c) Carbon dioxide + Water + Energy
(d) Pyruvate
11. The longest fiber on the cell body of a neuron is called
(a) sheath
(b) cytoplasm
(c) axon
(d) dendrites
12. Vegetative propagation refers to the formation of new plants from
(a) stem, flowers and fruits
(b) stem, leaves and flowers
(c) stem, roots and flowers
(d) stem, roots and leaves
13. A section of DNA providing information for one protein is called—
(a) Nucleus
(b) Chromosomes
(c) Trait
(d) Gene
14. What is the order of the waste management hierarchy, from most to least favoured?
a) Prevention-Recycle-Reuse-Disposal
b) Prevention-Reuse-Disposal-Recycle
c) Prevention-Disposal-Reuse-Recycle
d) Prevention-Reuse-Recycle-Disposal
15. Two heater wires of equal length are first connected in series and then in parallel with a battery. The ratio of heat produced in the two cases is:
(a) 2: 1
(b) 1: 2
(c) 4: 1
(d) 1: 4
16. What amount of energy will be received by omnivorous if herbivorous is receiving 200KJ from the producer?
(a) 2KJ
(b) 20KJ
(c) 50KJ
(d) 100KJ
Question No. 17 to 20 consist of two statements – Assertion (A) and Reason (R). Answer these questions by selecting the appropriate option given below:
a) Both A and R are true, and R is the correct explanation of A.
b) Both A and R are true, and R is not the correct explanation of A.
c) A is true but R is false.
d) A is false but R is true.
17. Assertion (A): Zinc carbonate is heated strongly in the presence of air to form zinc oxide and carbon dioxide.
Reason (R): Calcination is the process in which a carbonate ore is heated strongly in the absence of air to convert it into metal oxide.
d) A is false but R is true.
18. Assertion (A): Pyruvate is a six-carbon molecule
Reason (R): It is prepared in the cytoplasm as the first step to cellular respiration.
d) A is false but R is true.
19. Assertion: Accumulation of variation in a species increases the chances of its survival in a changing environment.
Reason: Accumulation of heat resistance in some bacteria ensures their survival even when the temperature in environment rises too much.
b) Both A and R are true, and R is not the correct explanation of A.
20. Assertion: The crown fires are most destructive as they burn the tree top.
Reason: Due to crown fire the temperature of that area may rise up to 700 degrees Celsius.
a) Both A and R are true, and R is the correct explanation of A.
Check Complete CBSE Class 10 Science Study Material for Board Exam 2025
Section-B
Questions No. 21 to 26 are very short answer questions
21. A spherical mirror’s radius of curvature is 20 cm. Calculate the focal length.
Answer:
Curvature Center is C
f = C/2 = 20/2 = 10 cm.
22. For dispersion of light through a prism which colour has a maximum deviation?
OR
What is the least distance of distinct vision of a normal human eye?
Answer:
Violet has the maximum deviation for the dispersion of light through a prism.
Or
The least distance of distinct vision of a normal human eye is 25 cm.
23. Using Kulhads as disposable cups to serve tea in trains, proved to be a bad idea. Why?
Answer:
Yes, it is a bad idea because making Kulhads on large scales leads to the loss of topsoil.
24. Write two differences between real and virtual images.
Answer:
Here are two differences between a real image and a virtual image:
- Formation: A real image is formed when light rays actually meet after reflection or refraction, whereas a virtual image is formed when light rays appear to meet but do not actually converge.
- Projection: A real image can be projected onto a screen, while a virtual image cannot be projected and can only be seen through an optical device like a mirror or lens.
25. Why red colour is selected for danger signal lights?
OR
Give reason: The sky appears dark instead of blue to an astronaut.
Answer:
The wavelength of red colour is more and so, it is least scattered. It can be easily seen through a large distance.
Or
The sky appears dark to the astronaut as scattering does not take place at very high altitudes due to the absence of atmosphere.
26. A bulb is rated at 5.0 V, 100 mA. Calculate its resistance.
Answer:
Rating of the bulb,
V = 5 0. Volt.
I = 100 mA
I 100x10-3
I = 0.1 A
V = IR,
R=V/I
5.0/0.1= 50Ω
Section-C
Questions No. 27 to 33 are short answer questions
27. Take 3 g of barium hydroxide in a test tube, now add about 2 g of ammonium chloride and mix the contents with the help of a glass rod. Now touch the test tube from outside.
(i) What do you feel about touching the test tube?
(ii) State the inference about the type of reaction that occurred.
(iii) Write the balanced chemical equation of the reaction involved.
Answer:
(i) When barium hydroxide is added to ammonium chloride, the bottom of the test tube is found to be cooler.
(ii) It is an endothermic reaction.
(iii) Ba(OH)2 + 2NH4Cl → BaCl2 + 2NH4OH
28. Suggest a method of reduction for the following metals during their metallurgical processes:
(i) Metal ‘A’ which is one of the last, second or third position in the reactivity.
(ii) Metal ‘B’ which gives vigorous reaction even with water and air.
(iii) Metal ‘C’ which is kept in the middle of the activity series.
Answer:
(i) ‘A’ can be obtained by chemical reduction using carbon or carbon monoxide as a reducing agent.
(ii) ‘B’ can be obtained by electrolytic reduction.
(iii) ‘C’ can be reduced by reducing agents like ‘Al’.
29. Rohan joined the gym and after the first day of exercise, he felt pain and cramps in his muscles. Explain the cause of cramps after excessive physical exercise.
Answer:
During excessive physical exercise, aerobic respiration produces energy in our muscles. Anaerobic respiration provides muscles with some extra energy required under excessive physical activity. Glucose is broken down into lactic acid due to anaerobic respiration. The accumulation of lactic acid causes muscle cramps.
30. The figure given below represents certain phenomena pertaining to the nervous system.
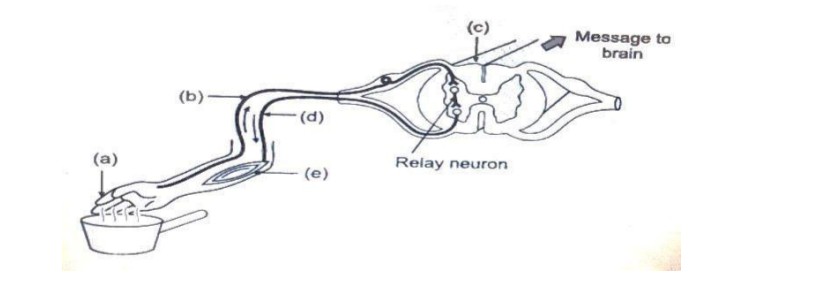
(i) What does the figure represent?
(ii) Name the parts labelled a, b, c, d and e.
(iii) Give the function of parts labelled a, b, d.
Answer:
(i) The figure represents the reflex arc.
(ii) a – Receptor,
b – Sensory neuron,
c – Spinal cord,
d – Motor neurons and
e – Effector.
Answer:
(iii) Receptor is a group of cells or organs that receives stimuli and converts them to an impulse. Sensory neuron carries the impulses from receptors to the central nervous system i.e., brain or spinal cord. The motor neuron carries impulses from the central nervous system to the effector organs.
31. In a monohybrid cross of tall Pea plants denoted by TT and short pea plants denoted by tt, Rahul obtained only tall plants (denoted by Tt) in F1 generation. However, in the F2 generation, she obtained both tall and short plants. Using the above information, explain the law of dominance.
Answer: The appearance of the trait of shortness in the F2 generation shows that the trait was present in F1 generation but was not expressed while the trait of tallness expressed itself. The trait which expresses itself in the presence of its contrasting form is called dominant. The other trait which is unable to express its effect in the presence of its contrasting trait is known as recessive.
32. What is overloading? State the causes of overloading.
Answer:
If more electrical appliances of a high-power rating like an electric iron box, electric cooker, electric heater, air conditioner etc., are switched on at the same time, they draw an extremely large current from the circuit is called overloading.
33. What are amphoteric oxides? Give examples.
Answer:
Amphoteric oxides are oxides that behave as both acidic and basic oxides. They can neutralize both acids and bases. They undergo a neutralization reaction to form water and salt when reacting with acid and form complex salts and water when reacting with base. Examples – Aluminium oxide (Al2O3) and Zinc Oxide (ZnO)
Section-D
Questions No. 34 to 36 are long answer questions.
- An aldehyde as well as a ketone can be represented by the same molecular formula, say C3H6O.
(i) Write their name and draw the structures.
(ii) State the relation between the two in the language of science.
Or
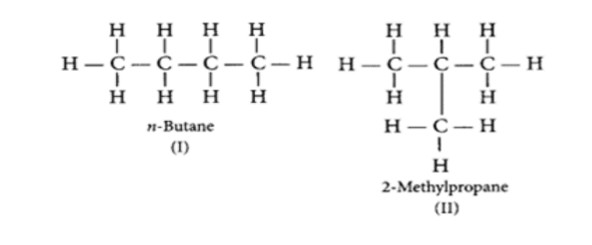
What is meant by isomers? Draw the structures of two isomers of butane (C4H10). Name three alkanes with molecular formulas which cannot show isomers.
Answer:
(i) The aldehyde and ketone are represented by the Molecular formula, C3H6O.
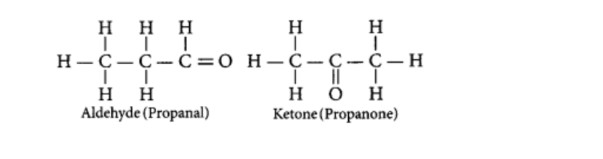
(ii) In the language of science, they are called isomers because both have the same molecular formula but different structural formulae (having different functional groups.)
Or
Isomers are those molecules which have the same molecular formula but different structural formula i.e., shows different properties. The structures of possible isomers of butane (C4H10) are:
The first three members of the alkane series are :
(i) CH4 (methane)
(ii) C2H6 (ethane)
(iii) C3H8 (propane)
- (i) Mention the names of flower parts which serve the same function as the following do in the animals.
(a) Testis (b) Sperm (c) Ovary
(ii) State the importance of Sepal/Calyx in the flower.
(iii) Which function is not interrupted if we cut the stigma of a flower?
Or
(i) Write any three functions of testes in the human male reproductive system.
(ii) Why are these located outside the abdominal cavity? Who is responsible for bringing about changes in appearance seen in boys at the time of puberty?
Answer:
(i) (a) Testis – anther, (b) Sperms – pollen grains, (c) Ovary- Ovary, (elaborate the function of the mentioned part in the flower.)
(ii) Sepal/Calyx protects the flower in the bud stage.
(iii) Pollination and Pollen germination cannot occur if we cut the stigma of flowers.
Or
(i) Functions of testes are:
1. To make male sex cells (or male gametes) called sperms.
2. To make the male sex hormone called testosterone.
3. The testes of a man lie in a small muscular pouch called the scrotum outside the abdominal cavity of the body. This is because sperm formation requires a lower temperature than the normal body temperature.
(ii) Being outside the abdominal cavity, the temperature of the scrotum is about 3°C lower than the temperature inside the body and thus the testes are provided an optimal temperature for the formation of sperms. The male sex hormone testosterone is responsible for bringing about changes in appearance seen in boys at the time of puberty.
36. (i) An object of size 7.0 cm is placed at 27 cm in front of a concave mirror of focal length 18 cm. At what distance from the mirror should a screen be placed, so that a sharp focused image can be obtained? Find the size and the nature of the image.
(ii) Radha needs an erect image from a concave mirror, Where does she need to place the objects, Draw the Ray diagram of a possible situation.
Or
A convex lens has a focal length of 10cm. At what distance from the lens should the object be placed so that it forms a real and inverted image 20cm away from the lens? What would be the size of the image formed if the object is 2cm high? With the help of a ray, the diagram shows the formation of the image by the lens in this case.
Answer:
(i) u = – 27 cm, f = – 18 cm. ho= 7.0 cm
1/v = 1/f- 1/u
1/v = -1/18 + 1/27 = -1/54
V = – 54 cm.
The screen must be placed at a distance of 54 cm from the mirror
in front of it.
hi/h0= v/u
hi/h0= v/u
hi/7 = +54/-27
hi = -2 x 7 = -14 cm.
Thus, the image is 14 cm in length and is an inverted image.
(ii) Radha needs to place the object between F (focus) and O
(Center of curvature).
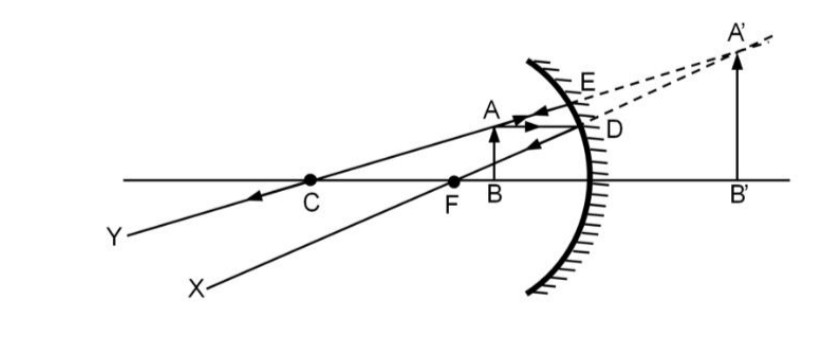
Or
Given, the focal length of the convex lens,
f=+10cm
Also, given the image formed is real and inverted with the
image distance as 20cm.
v=+20cm
From the lens formula, we have:
1f=1/v−1/u
1/10=1/20−1/u
1/u=1/20−1/10
1/u= −1/20
u=−20cm
∴The object is placed at a distance of 20cm.
Magnification is given as,
m= −v/u
m= −20/(−20)
m= +1
Also, magnification is given by, m=Height of the image
Height of the object.
∴m=Height of the image/2
1= height of the image/2
Height of the image=2cm
Thus, the image is of the same size as that of the object and it is real and inverted.
The ray diagram represents the formation of the image by the lens in this case:
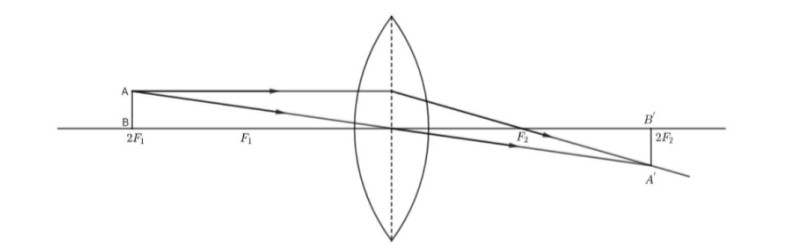
It is observed that the image is formed at 2F2 with the object placed at 2F1.
SECTION - E
Questions No. 37 to 39 are case-based/data-based questions with 2 to 3 short sub-parts. Internal choice is provided in one of these sub-parts.
37. The food that we eat gets broken down into simpler compounds for absorption. There are various enzymes and hormones secreted by various glands and cells of the digestive system, which facilitate the digestion of food. The undigested food is expelled out of the body through the anus. The main organs of the digestive system include the mouth, pharynx, oesophagus, stomach, small and large intestine, rectum and anus. There are various types of digestive glands present, e.g. salivary glands, pancreas, liver, etc.
1. The enzymes present in pancreatic juice are
(a) Amylase, Trypsinogen, Peptidase, Rennin
(b) Trypsinogen, Lipase, Amylase, Pro-carboxypeptidase
(c) Peptidase, Pepsin, Amylase, Rennin
(d) Maltase, Amylase, Trypsinogen, Pepsin
2. Which gland produces bicarbonate ions and Trypsin:
(a) Stomach and liver
(b) Liver and Pancreas
(c) Intestine and Liver
(d) Salivary gland and Intestine
3. Infants’ gastric juice contains
(a) nuclease, pepsinogen, lipase
(b) maltase, pepsinogen, rennin
(c) amylase, rennin, pepsinogen
(d) pepsinogen, lipase, rennin
4. The absorption of fructose by intestinal mucosa is
(a) co-transport mechanism
(b) simple diffusion
(c) facilitated transport
(d) active transport
Answer:
1. (b) Trypsinogen, Lipase, Amylase, Pro-carboxypeptidase
2. (b) Liver and Pancreas
3. (d) pepsinogen, lipase, rennin
4. (c) facilitated transport
38. Silver articles become black after some time when exposed to air. This is because it reacts with sulphur in the air to form a coating of silver sulphide.
Copper reacts with moist carbon dioxide in the air slowly loses its shiny brown surface and gains a green coat. This green substance is copper carbonate.
Iron when exposed to moist air for a long time acquires a coating of a brown flaky substance called rust. Let us find out the conditions under which iron rusts.
1. Select the correct balanced chemical reaction that occurs during the rusting of iron.
(a). 2Fe(s) + 2O2(g) + 2H2O(l) Fe2O3. X 3H2O
(b). 2Fe(s) + 2O2(g) + 2H2O(l) 2Fe2O3. X 2H2O
(c). Fe(s) + 3O2(g) + 2H2O(l) Fe2O3. X 3H2O
(d). 2Fe(s) + 2O2(g) + 3H2O(l) 2Fe2O3. X 2H2O
2. Select the method which is not useful to prevent the corrosion of metals.
(i) Galvanization
(ii) Painting & oiling
(iii) Anodizing or making alloys
(a) Only i
(b) Only ii
(c) i and iii both
(d) i, ii, and iii all
3. Amalgam belongs to metal-
(a) Gold
(b) Platinum
(c) Mercury
(d) Zinc
Or
Galvanization belongs to metal
(a) Gold
(b) Platinum
(c) Mercury
(d) Zinc
Answer:
1. (a). 2Fe(s) + 2O2(g) + 2H2O(l) Fe2O3. X 3H2O
2. (d) i, ii, and iii all
3. (c) Mercury
39. Observe the given line diagram of the electric circuit and answer the following questions.
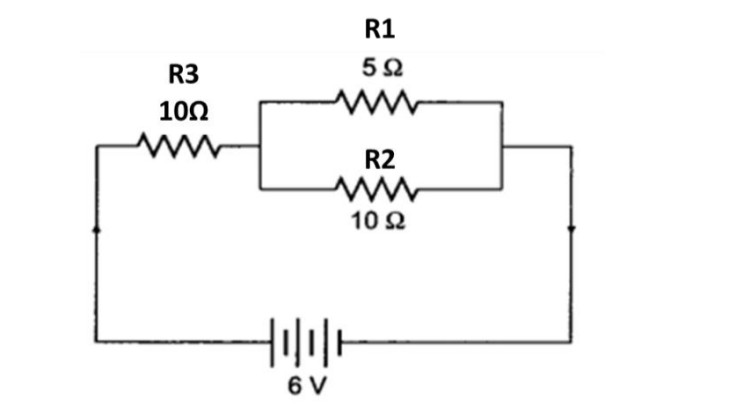
1. What is the total resistance of R1 and R2 only?
(a) 10/3Ω
(b) 3/10Ω
(a) 15Ω
(b) 2Ω
2. What will be total resistance if all the resistance readjusts to joint
in a series form.
(a) 15Ω
(b) 12Ω
(a) 17Ω
(b) 25Ω
3. Calculate the total resistance of the circuit if resistance R1 and R3 are exchanged to each other.
Or
Calculate the total resistance of the circuit if resistance R2 and R3 are exchanged with each other.
Answer:
1. (a) 10/3Ω
2. (b) 25Ω
3. Calculate the resistance after put the value R1=10Ω and R3=5Ω
The calculated answer is 10Ω
Or
Calculate the resistance after putting the value R2=10Ω and R3=10Ω
The calculated answer is 25/3Ω
To download the complete question paper in the pdf format, click on the link below:
| Download CBSE Class 10 Science Sample Paper Solutions Download PDF |
Comments
All Comments (0)
Join the conversation