The CBSE Class 12 Chemistry Exam 2025 is scheduled for February 27, and with the exam approaching, focused revision is essential for scoring high marks. To help students maximize their performance, our subject experts have compiled a list of sure-shot questions based on the latest CBSE syllabus and exam pattern. These carefully selected questions cover high-weightage chapters and frequently asked problems, ensuring a strategic and efficient revision.
Check: CBSE Class 12th Chemistry Syllabus for Board Exam 2025
Key Features of Class 12 Chemistry Sure-Shot Questions:
Expert-Curated Questions – Based on previous years' trends and exam analysis.
Covers High-Weightage Topics – Focuses on important concepts likely to appear in the exam.
Based on Latest CBSE Exam Pattern – Ensures alignment with the CBSE Class 12 Chemistry 2025 syllabus.
Includes Tricky & Application-Based Questions – Helps in improving problem-solving skills.
Boosts Confidence & Time Management – Ideal for last-minute practice before the board exam.
Practice these expert-selected questions now to enhance your preparation and secure top scores in the CBSE Class 12 Chemistry Board Exam 2025.
Read: Chemical Reactions for Class 12 Chemistry: Quick Revision
CBSE Class 12 Important Questions for Board Exam 2025
1. Define the following terms :
(a) Molecularity of reaction
(b) Complex reaction
2. Calculate the molar mass of a compound when 6.3 g of it is dissolved in 27 g of chloroform to form a solution that has a boiling point of 68.04 °C. The boiling point of pure chloroform is 61.04 °C and K, for chloroform is 3.63 °C kg mol-l
3. Write the stepwise mechanism of nucleophilic addition reactions in the carbonyl compounds.
OR
(b) How will you convert the following :
(i) Toluene to benzoic acid.
(ii) Ethanol to 3-Hydroxybutanal
4. (a) What happens when Glucose reacts with HI ? Write chemical equation.
(b) Which type of bond holds a DNA double helix together ?
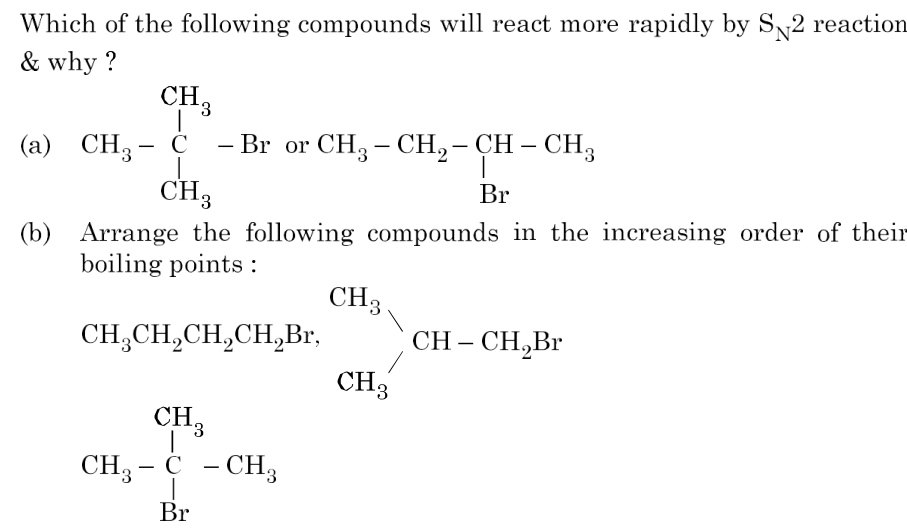
5. 
6. (a) Give chemical tests to distinguish between the following pairs of compounds:
(i) Phenol and Benzoic acid
(ii) Propanal and Propanone
(b) Which one of the given compounds is a stronger acid and why ?
![]()
7. 24. Show that the time required for 99.9% completion in a first order reaction is 10 times of half-life (t12) of the reaction [log 2 = 0.3010, log 10 = 1].
8. Give reasons for the following :
(1) Methyl amine is more basic than aniline.
(2) Aniline readily reacts with bromine water to give 2, 4, 6-tribromoaniline.
(3) Primary amines have higher boiling points than tertiary amines.
9. 33. Attempt any five of the following :
(a) Why Zine is not regarded as a transition element ?
(b) What is Lanthanoid contraction ?
(c) Why is first ionization enthalpy of chromium lower than that of Zn ?
(d) Why are transition elements good catalysts?
(e) Compounds of transition metals are generally coloured. Give reason.
(t) Out of KMnO, and K,MnO,, which one is paramagnetic and why ?
(g) Complete the following ionic equation :

10. An amide 'A' with molecular formula C-H,ON undergoes Hoffmann Bromamide degradation reaction to give amine 'B'. B' on treatment with nitrous acid at 273-278 K form 'C' and on treatment with chloroform and ethanolic potassium hydroxide forms 'D'. 'C' on. treatment, with ethanol gives E'. Identify 'A', B'. 'C' D' and 'E and write the sequence of chemical equations.
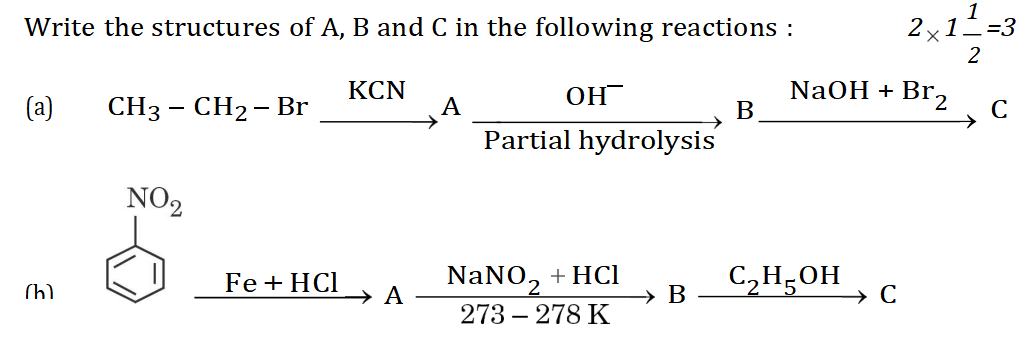
11. 
12. The vapour pressure of a solvent at 283 K is 100 mm Hg. Calculate the vapour pressure of a dilute solution containing 1 mole of a strong electrolyte AB in 50 moles of the solvent at 283 K (assuming complete dissociation of solute AB).
13. Give the equations of reactions for the preparation of: (any three)
(a)Phenol from chlorobenzene
(b) Salicylaldehyde from phenol
(c)2-Methoxyacetophenone from anisole
(d)Picric acid from phenol
14. Write the reaction involved in the following :
(a)Wolff-Kishner reduction
(b)Decarboxylation reaction
(c)Cannizzaro reaction
15. (i) The resistance of 0 05 M CH COOH solution is found to be 100 ohm. If the cell constant is 0 0354 cm-1 , calculate the molar conductivity of the acetic acid solution.
(ii) State Faraday's first law of electrolysis. How much charge in Faraday is required for the reduction of 1 mol of MnO4 to Mn2+ ?
Important*
CBSE Class 12th Chemistry Exam 2025: Last-Minute Writing Tips for
CBSE Class 12 Chemistry Sample Paper By Experts for Board Exam 2025
Top 50 MCQs for CBSE Class 12 Chemistry Exam 2025
Also Read:
- Periodic Table of Elements
- Atomic Mass And Molecular Mass of Elements
- Atomic Number and Mass Number
- Chemical Formula and Molecular Structure of Benzene
Comments
All Comments (0)
Join the conversation