In this article, we are providing you the MCQs from CBSE Class 10 Science Chapter 2: Acids, Bases and Salts. All the questions are provided with accurate answers. This set of questions will give the class 10 students a chance to test their knowledge of concepts and help them get prepared for the upcoming CBSE Exam. Check below the questions and answers for quick revision of fundamental concepts.
MCQs from CBSE Class 10 Science Chapter 2: Acids, Bases and Salts
1. Some fruits like mango, lemon, raw grapes, orange, etc., have a sour taste due to the presence of:
a. Acetic acid
b. Citric acid
c. Lactic acid
d. Oxalic acid
Answer. b. Citric acid
2. Zinc granules on treating with an acid X, form the zinc sulphate (ZnSO4) salt along with the evolution of a gas Y which burns with a pop sound when brought near to a burning candle. Identify the acid X and gas evolved Y.
a. X- Sulphuric acid and Y- Oxygen gas
b. X- Hydrochloric acid and Y- Oxygen gas
c. X- Sulphuric acid and Y- Hydrogen gas
d. X- Hydrochloric acid and Y- Hydrogen gas
Answer. c. X- Sulphuric acid and Y- Hydrogen gas
3. The figure given below represents the experiment carried out between conc. sulphuric acid and sodium chloride, which react with each other to form HCl gas.
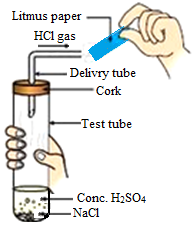
Blue litmus paper is brought near the mouth of the delivery tube to check the presence of HCl acid but no change is observed in the color of litmus paper because:
a. The litmus paper used is dry
b. The litmus paper used is moist
c. Blue litmus paper does not change its color with an acid
d. The litmus paper is kept very close to the mouth of the delivery tube
Answer. a. The litmus paper used is dry
4. Which of the following phenomena occur, when a small amount of acid is added to water?
i. Ionisation
ii. Neutralisation
iii. Dilution
iv. Salt formation
- (i) and (ii)
- (i) and (iii)
- (ii) and (iii)
- (ii) and (iv)
Answer. b. (i) and (iii)
5. Which of the following indicators turn red in an acidic solution?
i. Phenolphthalein
ii. Litmus
iii. Turmeric
iv. Methyl orange
Choose the correct option:
- (i) and (ii)
- (ii) and (iii)
- Only (ii)
- (ii) and (iv)
Answer. d. (ii) and (iv)
6. Dilute acid does not produce carbon dioxide on being treated with:
a. Marble
b. Lime
c. Baking soda
d. Limestone
Answer. b. Lime
7. The sample of soil from a particular place was tested for its pH value. It came out to be 5. Which one of the following should be added to the soil to make it suitable for the plant growth?
i. Calcium chloride
ii. Calcium Hydroxide
iii. Calcium oxide
Choose the correct option:
- Both (i) and (ii)
- Both (ii) and (iii)
- Only (i)
- Only (iii)
Answer. b. Both (ii) and (iii)
Also Check: CBSE Class 10 Science MCQs from Chapter 1: Chemical Reactions & Equations
8. Identify the products of the following reaction:
![]()
a. Calcium hydrogencarbonate and chlorine gas
b. Calcium chloride and water
c. Calcium oxide, carbon dioxide and water
d. Calcium chloride, carbon dioxide and water
Answer. d. Calcium chloride, carbon dioxide and water
9. An ant’s sting can be treated with …………which will neutralise the effect of the chemical injected by the ant’s sting into our skin.
Choose the correct option from the following to be filled in the blank space:
a. Methanoic acid
b. formic acid
c. Baking soda
d. Caustic soda
Answer. c. Baking soda
10. In the following reaction, identify the salt formed
NH4OH (aq) + H2SO4 (aq) → _____ + 2H2O (l)
a. NH4NO3
b. (NH4)2SO4
c. (NH4)3PO4
d. (NH4)2S
Answer. b. (NH4)2SO4
11. Which of the following salt will give acidic solution when dissolved in water?
a. NH4Cl
b. NaCl
c. Na2CO3
d. CH3COONa
Answer. a. NH4Cl
12. Bleaching powder is used as a disinfectant for water to:
a. Make water tastier
b. Remove all the dirt from water
c. Make water germ-free
d. Make water clear
Answer. c. Make water germ-free
13. Which among the following represents the chemical formula for ‘Plaster of Paris’?
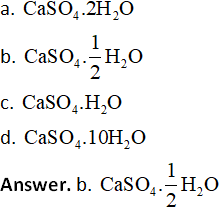
14. Which one of the following salts will dissolve in water to form an alkaline solution?
a. Potassium carbonate
b. Sodium chloride
c. Sodium carbonate
d. Potassium sulphate
Answer. a. Potassium carbonate
15. Copper sulphate crystals when heated strongly, lose their water of crystallization to give anhydrous copper sulphate accompanied by a change in color from:
a. Blue to green
b. Blue to white
c. Blue to sky blue
d. Blue to grey
Answer. b. Blue to white
MCQs on other chapters of class 10 can be checked from the following links:
Important MCQs from Chapter 1 Chemical Reactions & Equations
Important MCQs from Chapter 2 Acids, Bases and Salts
Important MCQs from Chapter 3 Metals and Non-Metals
Important MCQs from Chapter 4 Carbon and its Compounds
Important MCQs from Chapter 5 Periodic Classification of Elements
MCQs on the remaining chapters can be availed from the following link:
CBSE Class 10 Science Important MCQs: All Chapters
Also check the following links to explore some other useful articles to prepare in an effective manner for the changed pattern for CBSE Class 10 Science Exam 2020 and increase your chances to score more than 90% marks in the exam:
CBSE Class 10 Science Important Questions and Answers
CBSE Class 10 Science Solved Previous Year Question Papers
Comments
All Comments (0)
Join the conversation